Students & Educators —Menu
- Educational Resources
- Educators & Faculty
- Standards & Guidelines
- Periodic Table
- Adventures in Chemistry
- Landmarks Directory
- Frontiers of Knowledge
- Medical Miracles
- Industrial Advances
- Consumer Products
- Cradles of Chemistry
- Nomination Process
- Science Outreach
- Publications
- ACS Student Communities
- LEADS Conference
- ChemMatters
- Chemistry Outreach Activities
- National Chemistry Week
- U.S. National Chemistry Olympiad
- You are here:
- American Chemical Society
- Students & Educators
- Explore Chemistry
- Chemical Landmarks
- Joseph Priestley, Discoverer of Oxygen

Joseph Priestley and the Discovery of Oxygen
International historic chemical landmark.
Dedicated August 1, 1994, at the Joseph Priestley House in Northumberland, Pennsylvania, USA, and August 7, 2000, at Bowood House in Wiltshire, UK.
Priestley House Commemorative Booklet (PDF)
Bowood House Commemorative Booklet (PDF)
- En español: Joseph Priestley y el descubrimiento del oxígeno
Landmark Lesson Plan : Joseph Priestley, Discoverer of Oxygen
When Joseph Priestley discovered oxygen in 1774, he answered age-old questions of why and how things burn. An Englishman by birth, Priestley was deeply involved in politics and religion, as well as science. When his vocal support for the American and French revolutions made remaining in his homeland dangerous, Priestley left England in 1794 and continued his work in America until his death.
About Joseph Priestley
Understanding the composition of air, oxygen and other discoveries in england, religion and emigration to america, further reading, landmark designation and acknowledgments, cite this page.

Some 2,500 years ago, the ancient Greeks identified air — along with earth, fire and water — as one of the four elemental components of creation. That notion may seem charmingly primitive now. But it made excellent sense at the time, and there was so little reason to dispute it that the idea persisted until the late 18th century. It might have endured even longer had it not been for a free-thinking English chemist and maverick theologian named Joseph Priestley.
Priestley (1733-1804) was hugely productive in research and widely notorious in philosophy. He invented carbonated water and the rubber eraser, identified a dozen key chemical compounds, and wrote an important early paper about electricity. His unorthodox religious writings and his support for the American and French revolutions so enraged his countrymen that he was forced to flee England in 1794. He settled in Pennsylvania, where he continued his research until his death.
The world recalls Priestley best as the man who discovered oxygen, the active ingredient in our planet's atmosphere. In the process, he helped dethrone an idea that dominated science for 23 uninterrupted centuries: Few concepts "have laid firmer hold upon the mind," he wrote, than that air "is a simple elementary substance, indestructible and unalterable."
In a series of experiments culminating in 1774, Priestley found that "air is not an elementary substance, but a composition," or mixture, of gases. Among them was the colorless and highly reactive gas he called "dephlogisticated air," to which the great French chemist Antoine Lavoisier would soon give the name "oxygen."
It is hard to overstate the importance of Priestley's revelation. Scientists now recognize 92 naturally occurring elements-including nitrogen and oxygen, the main components of air. They comprise 78 and 21 percent of the atmosphere, respectively.
Back to top

In the mid-18th century, the concept of an element was still evolving. Researchers had distinguished no more than two dozen or so elements, depending on who was doing the counting. It wasn't clear how air fit into that system. Nobody knew what it was, and researchers kept finding that it could be converted into such a variety of forms that they routinely spoke of different "airs."
The principal method for altering the nature of air, early chemists learned, was to heat or burn some compound in it. The second half of the 1700s witnessed an explosion of interest in such gases. The steam engine was in the process of transforming civilization, and scientists of all types were fascinated with combustion and the role of air in it.
British chemists were especially prolific. In 1754, Joseph Black identified what he called "fixed air" (now known to be carbon dioxide) because it could be returned, or fixed, into the sort of solids from which it was produced. In 1766, a wealthy eccentric named Henry Cavendish produced the highly flammable substance Lavoisier would name hydrogen, from the Greek words for "water maker."
Finally in 1772, Daniel Rutherford found that when he burned material in a bell jar, then absorbed all the "fixed" air by soaking it up with a substance called potash, a gas remained. Rutherford dubbed it "noxious air" because it asphyxiated mice placed in it. Today, we call it nitrogen.
But none of those revelations alone tells the whole story. The next major discovery would come from a man whose early life gave no indication that he would become one of the greatest experimental chemists in history.
Bubbling Beverages
In 1767, Priestley was offered a ministry in Leeds, Englane, located near a brewery. This abundant and convenient source of "fixed air” — what we now know as carbon dioxide — from fermentation sparked his lifetime investigation into the chemistry of gases. He found a way to produce artificially what occurred naturally in beer and champagne: water containing the effervescence of carbon dioxide. The method earned the Royal Society's coveted Copley Prize and was the precursor of the modern soft-drink industry.
Joseph Priestley was born in Yorkshire, the eldest son of a maker of wool cloth. His mother died after bearing six children in six years. Young Joseph was sent to live with his aunt, Sarah Priestley Keighley, until the age of 19. She often entertained Presbyterian clergy at her home, and Joseph gradually came to prefer their doctrines to the grimmer Calvinism of his father. Before long, he was encouraged to study for the ministry. And study, as it turned out, was something Joseph Priestley did very well.
Aside from what he learned in the local schools, he taught himself Latin, Greek, French, Italian, German and a smattering of Middle Eastern languages, along with mathematics and philosophy. This preparation would have been ideal for study at Oxford or Cambridge, but as a Dissenter (someone who was not a member of the Church of England) Priestley was barred from England's great universities. So he enrolled at Daventry Academy, a celebrated school for Dissenters, and was exempted from a year of classes because of his achievements.
After graduation, he supported himself, as he would for the rest of his life, by teaching, tutoring and preaching. His first full-time teaching position was at the Dissenting Academy in Warrington. (Although obviously brilliant, original, outspoken and, by one report, of "a gay and airy disposition," Priestley had an unpleasant voice and a sort of stammer. That he made a living through lectures and sermons is further evidence of his extraordinary nature.)
In 1762, he was ordained and married Mary Wilkinson, the daughter of a prominent iron-works owner. She was, he noted, "of an excellent understanding, much improved by reading, of great fortitude and strength of mind, and of a temper in the highest degree affectionate and generous; feeling strongly for others and little for herself."
Priestley traveled regularly to London, and became acquainted with numerous men of science and independent thought, including an ingenious American named Benjamin Franklin, who became a lifelong friend. Franklin encouraged Priestley in his research, one result of which was The History and Present State of Electricity . For that work, and his growing reputation as an experimenter, Priestley was made a Fellow of the Royal Society in 1766.
The History book was too tough for a popular audience, and Priestley determined to write a more accessible one. But he could find no one to create the necessary illustrations. So, in typical fashion, he taught himself perspective drawing. Along the way, he made many mistakes, and discovered that India rubber would erase lead pencil lines — a fact he mentioned in the preface.
By the age of 34, Priestley was a well-established and respected member of Britain's scientific community. He was still paying a price for his religious nonconformity, however. When the explorer Captain James Cook was preparing for his second voyage, Priestley was offered the position of science adviser. But the offer was rescinded under pressure from Anglican authorities who protested his theology, which was evolving into a strongly Unitarian position that denied the doctrine of the trinity.
In retrospect, the Cook affair may have been all for the best. In 1773, the Earl of Shelburne asked Priestley to serve as a sort of intellectual companion, tutor for the earl's offspring, and librarian for his estate, Bowood House. The position provided access to social and political circles Priestley could never have gained on his own, while leaving ample free time for the research that would earn him a permanent place in scientific history.
He systematically analyzed the properties of different "airs" using the favored apparatus of the day: an inverted container on a raised platform that could capture the gases produced by various experiments below it. The container could also be placed in a pool of water or mercury, effectively sealing it, and a gas tested to see if it would sustain a flame or support life.
In the course of these experiments, Priestley made an enormously important observation. A flame went out when placed in a jar in which a mouse would die due to lack of air. Putting a green plant in the jar and exposing it to sunlight would "refresh" the air, permitting a flame to burn and a mouse to breathe. Perhaps, Priestley wrote, "the injury which is continually done by such a large number of animals is, in part at least, repaired by the vegetable creation." Thus he observed that plants release oxygen into the air — the process known to us as photosynthesis.
On August 1, 1774, he conducted his most famous experiment. Using a 12-inch-wide glass "burning lens," he focused sunlight on a lump of reddish mercuric oxide in an inverted glass container placed in a pool of mercury. The gas emitted, he found, was "five or six times as good as common air." In succeeding tests, it caused a flame to burn intensely and kept a mouse alive about four times as long as a similar quantity of air.
Priestley called his discovery "dephlogisticated air" on the theory that it supported combustion so well because it had no phlogiston in it, and hence could absorb the maximum amount during burning. (The year before, Swedish apothecary Carl Wilhelm Scheele isolated the same gas and observed a similar reaction. Scheele called his material "fire air." But his findings were not published until 1777.)
Whatever the gas was called, its effects were remarkable. "The feeling of it in my lungs," Priestley wrote, "was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell but that in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it."
Phlogiston and Fire
In the mid-18th century, the most pressing issue in chemistry and physics was to determine what exactly happens when something burns. The prevailing theory was that flammable materials contained a substance called “phlogiston” (from the Greek word for burn) that was released during combustion.
The theory held that when a candle burned, for example, phlogiston was transferred from it to the surrounding air. When the air became saturated with phlogiston and could contain no more, the flame went out. Breathing, too, was a way to remove phlogiston from a body. A typical test for the presence of phlogiston was to place a mouse in a container and measure how long it lived. When the air in the container could accept no more phlogiston, the mouse would die.
The 18 th century scientist Antoine Lavoisier disproved the existence of phlogiston and helped to form the basis of modern chemistry using Joseph Priestley’s discovery of oxygen.
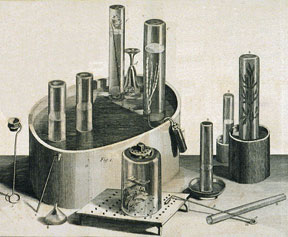
As luck would have it, Lord Shelburne was setting off on a trip to the European continent and took Priestley along. In France, Priestley met Lavoisier and described his discovery. It turned out to be the clue Lavoisier needed to develop his theory of chemical reactions — the "revolution" in chemistry that would finally dispel the phlogiston theory. Burning substances, Lavoisier argued, did not give off phlogiston; they took on Priestley's gas, which Lavoisier called "oxygen" from the Greek word for acid-maker.
By then, however, Priestley had returned to England, where he escalated his support for the American Revolution and for highly unorthodox religious views. Those positions were a source of embarrassment for Lord Shelburne. Priestley left his service in 1780, moving to Birmingham and taking a position as head of a liberal congregation called New Meeting.
His new location brought him into contact with numerous luminaries including Erasmus Darwin, grandfather of Charles, the great architect of evolutionary theory. James Watt and Matthew Boulton — who were about to transform society with their steam engine — were there, as was Josiah Wedgwood, the famous potter, who supported Priestley's chemical experiments. Birmingham also boasted a distinguished scientific discussion group, the Lunar Society, which met on nights of a full moon so that the members could see their way home.
Priestley's encouragement of the French Revolution, together with his increasingly controversial theology and attacks on the doctrine of the trinity, eventually became too notorious for safety. In 1791, an alcohol-fueled mob of royalists burned the New Meeting house, and then Priestley's home. The scientist and his family barely escaped. They fled to London, but eventually it proved no safer. Priestley's sons could not find work and emigrated to Pennsylvania, where they hoped to found a center for free-thinking Englishmen.
Finally Joseph and Mary followed them, setting sail for America on April 8, 1794. Priestley turned down the offer of a teaching position at the University of Pennsylvania in Philadelphia, and instead built a house in the remote hamlet of Northumberland to be near his sons. The area was decidedly rustic.
There Priestley continued his research, isolating carbon monoxide (which he called "heavy inflammable air") and founding the Unitarian Church in the United States. For the most part, he led a quiet and reflective life — especially after his friend Thomas Jefferson was elected president in 1800.
During his final trip to Philadelphia, he told the Philosophical Society that "having been obliged to leave a country which has been long distinguished by discoveries in science, I think myself happy by my reception in another which is following its example, and which already affords a prospect of its arriving at equal eminence." His words proved prophetic. A colloquium held on the centennial of Priestley's discovery of oxygen led to the founding of the American Chemical Society —today the world's largest scientific society — in 1876.
On February 3, 1804, Priestley began an experiment, but found himself too weak to continue. He went to his bed in his library, never again to emerge. On February 6, he summoned one of his sons and an assistant. He dictated some changes in a manuscript. When he was satisfied with the revisions, he said "That is right. I have now done." Minutes later he died painlessly, ending what Jefferson called "one of the few lives precious to mankind."
- The Joseph Priestley House (Pennsylvania Historical & Museum Commission)
- Joseph Priestley (Chemical Heritage Foundation)

Landmark Designation
The American Chemical Society dedicated the Joseph Priestley House a National Historic Chemical Landmark on August 1, 1994. The plaque commemorating the event reads:
Joseph Priestley (1733-1804) — Unitarian minister, teacher, author, natural philosopher, discoverer of oxygen, and friend of Benjamin Franklin and Thomas Jefferson — supervised the construction of this house and laboratory from 1794 to 1798, then lived and worked here until his death in 1804. His library of some 1,600 volumes and his chemical laboratory, where he first isolated carbon monoxide, were probably the best in the country at that time. As suggested by Edgar Fahs Smith in 1920, the Joseph Priestley House has become "a Mecca for all who would look back to the beginnings of chemical research" in America.
The American Chemical Society and the Royal Society of Chemistry dedicated the Discovery of Oxygen by Joseph Priestley an International Historic Chemical Landmark on August 7, 2000, in Wiltshire, UK. The plaque commemorating the event reads:
Joseph Priestley (1733-1804) — Unitarian minister, teacher, author, and natural philosopher — was the Earl of Shelburne's librarian and tutor to his sons. In this room, then a working laboratory, Priestley pursued his investigations of gases. On 1 August 1774 he discovered oxygen. Twenty years later he emigrated to America where he continued his research at his home and laboratory in Northumberland, Pennsylvania.
Acknowledgments:
Adapted for the internet from “The Joseph Priestley House,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 1994 and “Bowood House,” produced by the National Historic Chemical Landmarks program of the American Chemical Society and the Royal Society of Chemistry in 2000.
American Chemical Society International Historic Chemical Landmarks. Discovery of Oxygen by Joseph Priestley. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/josephpriestleyoxygen.html (accessed Month Day, Year).
Back to Landmarks Main Page
Learn more: About the Landmarks Program
Take action: Nominate a Landmark and Contact the NHCL Coordinator
Teach: Landmark Lesson Plans

Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A paradigm of fragile Earth in Priestley's bell jar
Daniel martin, andrew thompson, iain stewart, edward gilbert, katrina hope, grace kawai, alistair griffiths.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2012 Mar 14; Accepted 2012 Sep 4; Collection date 2012.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Photosynthesis maintains aerobic life on Earth, and Joseph Priestly first demonstrated this in his eighteenth-century bell jar experiments using mice and mint plants. In order to demonstrate the fragility of life on Earth, Priestley's experiment was recreated using a human subject placed within a modern-day bell jar.
A single male subject was placed within a sealed, oxygen-depleted enclosure (12.4% oxygen), which contained 274 C 3 and C 4 plants for a total of 48 h. A combination of natural and artificial light was used to ensure continuous photosynthesis during the experiment. Atmospheric gas composition within the enclosure was recorded throughout the study, and physiological responses in the subject were monitored.
After 48 h, the oxygen concentration within the container had risen to 18.1%, and hypoxaemia in the subject was alleviated (arterial oxygen saturation rose from 86% at commencement of the experiment to 99% at its end). The concentration of carbon dioxide rose to a maximum of 0.66% during the experiment.
Conclusions
This simple but unique experiment highlights the importance of plant life within the Earth's ecosystem by demonstrating our dependence upon it to restore and sustain an oxygen concentration that supports aerobic metabolism. Without the presence of plants within the sealed enclosure, the concentration of oxygen would have fallen, and carbon dioxide concentration would have risen to a point at which human life could no longer be supported.
Keywords: Atmosphere, Carbon dioxide, Oxygen, Hypoxia, Photosynthesis, Plants
The Earth supports a fragile ecosystem, and its inhabitants depend for their survival upon complex interactions between them, which have developed over billions of years. Imbalance of one component in this bionetwork can have far-reaching effects on organisms whose existence relies upon the presence of other species. Despite the ability to alter their environment in diverse ways, humans are reliant for their survival upon an element derived primarily from plants and produced by chlorophyll during photosynthesis, oxygen (O 2 ).
Photosynthesis is arguably the single most important chemical process on our planet, and the first colour images captured of Earth from space revealed the vast green hues of the landmasses supporting plant life, confirming its dominance within our ecosystem. Using energy from sunlight, chlorophyll strips electrons from water molecules, which then convert atmospheric carbon dioxide (CO 2 ) into carbon compounds, producing O 2 as a byproduct. Whilst mechanisms that use alternative naturally available compounds to release energy exist, the abundance of water on the surface of the Earth meant that photosynthesis rapidly became the foremost bio-energetic pathway on the planet. During the early era of chlorophyll photosynthesis, approximately 2,400 million years ago [ 1 ], the atmosphere was rich in CO 2 , whilst O 2 was scarce. As time progressed and photosynthetic species slowly overwhelmed the surface of the Earth, the concentration of O 2 rose and eventually reached levels we are accustomed to today.
In the early 1770 s, Joseph Priestley conducted a series of experiments that led to the discovery of the intimate relationship between plant and animal life [ 2 ]. In his principal experiment, Priestley placed a mouse within a sealed jar and observed it to eventually perish. When repeated with sprigs of mint within the jar, neither did the animal die ‘nor was it at all inconvenient to a mouse’ [ 2 ]. He had made the breakthrough that plants produce a substance which is life-giving to animals and then went on to describe ‘dephlogisticated air’, which, thanks to the French chemist Antoine Lavoisier, soon became known as ‘oxygen’. The story of photosynthesis was completed in 1779 when a Dutchman, Jan Ingenhousz, demonstrated that the process by which plants produce O 2 is dependent upon light.
We hypothesised that a human could survive within a sealed modern-day bell jar, even if the O 2 concentration within was significantly reduced from the outset, provided that it contained sufficient plant matter to generate O 2 and remove CO 2 via photosynthesis.
Formal ethical approval was not sought for this experiment as it was designed for the purpose of a television demonstration; consent was implied through the subject's involvement in the project and participation in the event. The Chair of the University College London Committee on the Ethics of Non-NHS Human Research approved this strategy. Prior to commencing the experiment, a full medical screening questionnaire was completed by the subject, and he was assessed by a physician with experience in high altitude and acute hypoxia research (DM). The protocol was explained to the subject in full, along with a description of the potential risks and safety measures in place. A standard resuscitation kit was available throughout the experiment, along with bottled supplemental oxygen. A physician trained in Advanced Life Support was also present outside the container throughout the experiment, with the ability to enter the container at any point should there be concerns regarding the welfare of the subject.
We constructed the first human recreation of Priestley's ‘mouse in a bell jar’ experiment to demonstrate the ability of plants to generate sufficient O 2 to sustain human life in an enclosed environment [ 3 ]. A healthy 47-year-old male was placed within a transparent airtight container measuring 2.0 × 2.5 × 6.0 m (30 m 3 , Figure 1 ), itself placed within the rainforest biome at the Eden Project, Cornwall, UK. A selection of plants known for their high photosynthetic yield (under certain environmental conditions) was placed within the container. Prior to the experiment, containerised plants were grown in a peat-free Eden Project Melcourt mix within a standard glasshouse at a relative humidity of 70% to 80% and temperature range of 15°C to 30°C. During this growing phase, the plants were watered with liquid nutrient feed at 20 ml/L (N 177 ppm, P 35 ppm, K 119 ppm, Ca 49 ppm, Mg 17 ppm, B 0.2 ppm, Cu 0.08 ppm, Fe 1.44 ppm, Mn 0.48 ppm, Mo 0.04 ppm and Zn 0.64 ppm). In total, 274 plants consisting of 18 different taxa were placed within the container, with 10,967 leaves (excluding Tillandsia usneoides ) and a total leaf area of 1,106,033 cm 2 (Table 1 ). A mixture of C 3 (ribulose diphosphate carboxylase utilising) and C 4 (phosphoenolpyruvate carboxylase utilising) plants were selected in order to maximise photosynthetic potential within the container. Several C 4 carbon fixation plants were grown, including Miscanthus x giganteus and Zea mays (maize), because they have advantages over C 3 plants, resulting in superior carbon-gaining capacities and photosynthetic efficiency [ 4 ]. During the experiment, the subject regularly irrigated the plants when deemed necessary from a water source within the container.

The sealed container with plants, the subject and external artificial lighting.
Taxa, number of leaves and leaf area of the plants placed within the container
Individual leaf areas were determined by tracing a leaf onto graph paper; the area of the petiole was not included within the calculations. From each individual plant, a subsample representing three small, three medium and three large leaves were harvested, and the mean of each of the three leaves was taken and used to provide a representative small, medium and large leaf area. The number of small, medium and large leaves in each individual plant was then counted, and the corresponding areas were used to estimate the total leaf surface area. a Total leaf area was calculated as both the upper and lower sides of the leaves. T. usneoides , a moss, was also placed within the container, but it was not possible to calculate leaf area.
In order to more clearly demonstrate oxygen production and highlight the effectiveness of photosynthesis in preserving human life, the environment within the container was rendered hypoxic at the start of the experiment. Three hypoxic generators (Hypoxico Everest Summit II, Hypoxico Inc, New York, NY, USA) were used to reduce the concentration of O 2 in the container. These devices consist of a molecular sieve system that uses zeolite to separate nitrogen from O 2 in the air and consequently provides a nitrogen-rich gas mixture to purge the atmosphere within the container. Connected to the container, and in conjunction with a one-way pressure relief valve, the hypoxic generators reduced the concentration of O 2 to 12.4% prior to commencing the experiment. Once the subject was sealed inside the container and safety procedures had been confirmed, the hypoxic generators were switched off and the one-way valves were closed. Artificial lighting (8 × 2,000 W systems; ARRI, Munich, Germany) was placed around the container externally and switched on at the beginning of the experiment. A split air-conditioning unit (Clima 16 HP Portable Air Conditioner, Toshiba, Tokyo, Japan) was used to maintain temperatures for optimal plant growth and comfort for the subject whilst ensuring a sealed atmosphere. The concentrations of O 2 and CO 2 within the container were monitored with a gas analyser (Aspida, Analox, London, UK) and plotted every hour along with temperature and humidity from a digital hygro-thermometer (Brannan, Cumbria, UK). The subject's heart rate and arterial O 2 saturation (SpO 2 ) were monitored continuously (Johnson and Johnson Dinamap MPS Monitor and Onyx 9500, Nonin, Plymouth, MN, USA); respiratory rate was recorded hourly by manual calculation.
The concentration of O 2 in the container rose throughout the experiment, peaking at 18.1% in the final hour (hour 48; Figure 2 ). The CO 2 concentration fluctuated depending on the subject's activity within the container (declining noticeably during sleep), but there was an overall rise that peaked at 0.66%, approximately halfway through the experiment (Figure 3 ). There was a diurnal variation in temperature (25.3°C to 28.4°C), and humidity varied between 57% and 87%. On entering the hypoxic container, the subject had a heart rate of 90 beats per minute, respiratory rate of 20 breaths per minute and SpO 2 of 86%. These figures returned to the subject's resting normal values as the concentration of O 2 rose within the container. The subject's final SpO 2 was 99% (Figure 4 ).
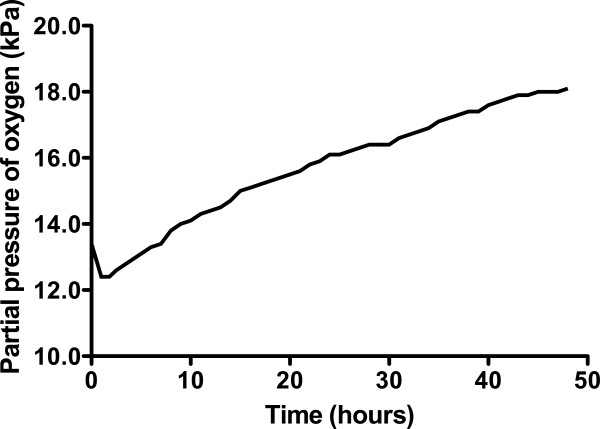
Change in oxygen concentration within the container over time.
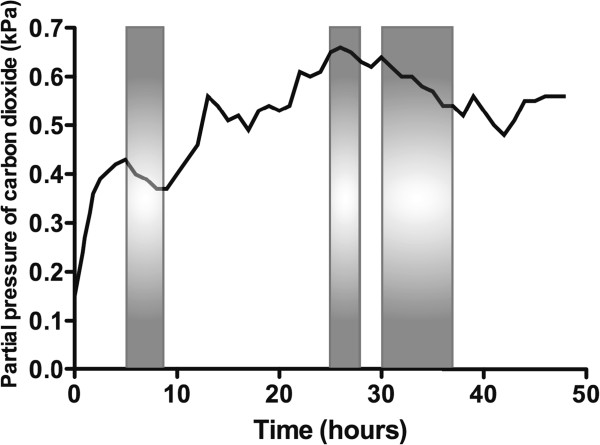
Change in carbon dioxide concentration within the container over time. The shaded areas are those during which the subject was sleeping.
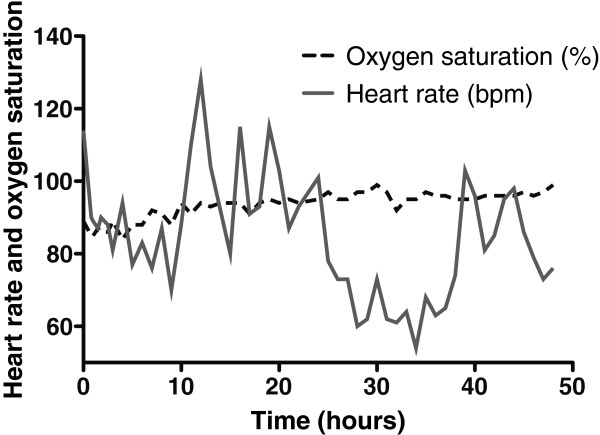
Changes in the subject's oxygen saturation and heart rate during enclosure within the container.
The design of the biological ecosystem in this study was such that human life was sustained for 48 h and the initial hypoxic environment restored to one of near-normal O 2 concentration. In the early 1990's, the ‘Biosphere 2’ experiment was conducted to explore the feasibility of self-sustaining biospheres in space. This grand design consisted of a 200 m 3 atmosphere within a dome that contained eight volunteers, which was designed to sustain them for 2 years [ 5 ]. However, the O 2 concentration within the biosphere dropped from 20.9% to 14.2% after 16 months, so additional O 2 had to be added to the atmosphere [ 6 ]. This decline was traced to a two-step process: firstly, there was O 2 loss to organic soil matter producing CO 2 , and secondly, the CO 2 was being captured by structural concrete to form calcium carbonate [ 5 ]. In the current experiment, the initial O 2 concentration of 12.4% (equivalent to approximately 4,500 m above sea level) resulted in a marked reduction in the subject's SpO 2 and represents an acute hypoxic exposure that is frequently associated with symptoms of altitude-related illness [ 7 ]. During the last few hours of the study, there was a small reduction in rate of the O 2 concentration rise, perhaps due to deterioration in the condition of the plants, noticeable towards the end of the experiment. Direct heating and excessive light exposure, arguably both present in this experiment, can lead to the denaturing of enzymes within chlorophyll [ 8 ]. There were fluctuations in CO 2 concentration throughout the study, with a tendency for it to rise as time progressed (Figure 3 ).
As well as providing an insight into the use of plants to maintain a self-sufficient biosphere, such as would be required on the surface of extra-terrestrial bodies without an atmosphere, our experiment highlights the detrimental effects of a markedly increased CO 2 concentration. CO 2 concentrations have altered dramatically over the course of the Earth's history [ 9 ], and there is much concern that levels are now rising at an alarming rate [ 10 ]. Under certain environmental conditions, increasing the ambient concentration of CO 2 can be beneficial, increasing photosynthetic activity, plant growth and yield [ 11 , 12 ]. Using CO 2 enrichment to increase plant growth and yield is now commonplace in commercial glasshouse crop production, with optimal levels being between 700 and 1,000 ppm [ 13 ]. However, in some species, super-elevated CO 2 concentrations (over 2,000 ppm) induces foliar symptoms of chlorosis and necrosis [ 14 , 15 ], and levels above 10,000 ppm are known to cause damage to young maize plants after 48 h in the form of ‘yellow streaks’ [ 16 ]. During this experiment, the CO 2 levels remained above 2,000 ppm and reached a maximum of 6,600 ppm, yet yellow streaks were observed on the maize plants by the end. It is possible that damage to the maize may have also reduced the photosynthetic yield and the production of O 2 towards the end of this experiment. This study, therefore, provides an insight into the use of plants to maintain a self-sufficient biosphere, such as would be required on the surface of extra-terrestrial bodies without an atmosphere, and the potentially detrimental effects of a dramatically increased CO 2 concentration.
This simple experiment is a humble reminder of the integral relationship between animal and plant life on Earth, in which the former owe their existence to the latter. Without the presence of plants within the sealed environment, the concentration of O 2 would have fallen and CO 2 concentration would have risen to a point at which human life could no longer be supported. Whilst O 2 sustains human life and plants maintain its level within the atmosphere with remarkable efficiency, the fundamental role of photosynthesis is arguably taken for granted. Deprived of plants, the subject within the container would have succumbed to the effects of severe hypoxaemia. The experiment reminds us of our total dependency upon plants, and the ecosystem in which they exist.
Competing interests
The author declares that they have no competing interests.
Authors’ contributions
AT conceived the idea, and the experiment was designed by AT, AG and DM. The experiment was conducted by DM, KH, EG, GK, AT and IS. Data were analysed by DM, and the manuscript was written by DM, AG and EG. All authors discussed the results and implications and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Contributor Information
Daniel Martin, Email: [email protected].
Andrew Thompson, Email: [email protected].
Iain Stewart, Email: [email protected].
Edward Gilbert, Email: [email protected].
Katrina Hope, Email: [email protected].
Grace Kawai, Email: [email protected].
Alistair Griffiths, Email: [email protected].
Acknowledgements
We would like to thank the BBC Scotland team for the ‘How to Make a Planet’ series and all the staff at the Eden Project in Cornwall who made this experiment possible.
- Blankenship RE. Origin and early evolution of photosynthesis. Photosynth Res. 1992;33:91–111. doi: 10.1007/BF00039173. [ DOI ] [ PubMed ] [ Google Scholar ]
- Priestley J. Experiments and Observations on Different Kinds of Air. W. Bowyer and J. Nichols, London; 1774. [ Google Scholar ]
- Water the plant, take a breath. Science. 2011;333:1685. [ Google Scholar ]
- Sage RF, Zhu XG. Exploiting the engine of C(4) photosynthesis. J Exp Bot. 2011;62:2989–3000. doi: 10.1093/jxb/err179. [ DOI ] [ PubMed ] [ Google Scholar ]
- Allen J, Nelson M. Overview and design biospherics and biosphere 2, mission one (1991–1993) Ecol Engin. 1999;13:15–29. doi: 10.1016/S0925-8574(98)00089-5. [ DOI ] [ Google Scholar ]
- Dempster WF. 23rd International conference on environmental systems: July 1993; Colorado. SAE International, Warrendale; 1993. Biosphere 2. System dynamics and observations during the initial two-year closure trial. [ Google Scholar ]
- Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345:107–114. doi: 10.1056/NEJM200107123450206. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bohning RH. Time course of photosyntesis in apple leaves exposed to continuous illumination. Plant Physiol. 1949;24:222–240. doi: 10.1104/pp.24.2.222. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. [ DOI ] [ PubMed ] [ Google Scholar ]
- Alley R, Berntsen T, Bindoff NL, Chen Z, Chidthaisong AF P, Gregory J, Hegel G, Heimann M, Hewitson B. Climate Change 2007: The Physical Science Basis, Summary for Policymakers. Intergovernmental Panel on Climate Change Secretariat, Geneva; 2007. [ Google Scholar ]
- Körner C. Biosphere responses to CO2 enrichment. Ecol Appl. 2000;10:1590–1619. [ Google Scholar ]
- Drake BG, Gonzalez-Meler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hanan JJ. Greenhouses. Advanced technology for protected horticulture. CRC Press, London; 1998. [ Google Scholar ]
- Ehret DL, Jolliffe PA. Leaf injury to bean plant grown in carbon dioxide enriched atmospheres. Can J Bot. 1985;63:2015–2020. [ Google Scholar ]
- van Berkel N. Injurious effects of high CO2 concentration on cucumber, tomato, chrysanthemum and gerbera. Acta Horticulturae. 1984;162:101–112. [ Google Scholar ]
- Schwarz M. Carbon toxicity in plants. Acta Horticulturae. 1999;481:685–688. [ Google Scholar ]
- View on publisher site
- PDF (889.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

How priestly lightened the candle without touching the belljar
In 1771 and 1772, joseph priestley performed a series of experiments that revealed the essential role of air in the growth of green plants. priestley observed that a candle burning in a closed space such as a bell jar, soon gets extinguished. similarly, a mouse would soon suffocate in a closed space. it was known that a candle placed in a sealed bell jar would eventually burn out and could not be relighted while still in the jar. in august 1771, joseph priestley, placed a mint plant into a transparent closed space with a candle that burned out the air until it soon went out. after 27 days, he relit the extinguished candle again. since there was no bright source of light available that time, priestly had to rely on the sun. he focused sunlight beams with a mirror onto the candle wick and was successful in relighting the candle from outside the bell jar without disturbing the experimental set up. now, we can use more sophisticated methods to light the candle like focusing light from a flood light through converging lens, or by an electrical spark. priestly discovered that the relit candle burned perfectly well in the air that previously would not support it..

Lavoisier and Priestly named the same gas ______ and __________ respectively.

- Open access
- Published: 04 September 2012
A paradigm of fragile Earth in Priestley's bell jar
- Daniel Martin 1 , 2 ,
- Andrew Thompson 3 ,
- Iain Stewart 4 ,
- Edward Gilbert 1 ,
- Katrina Hope 5 ,
- Grace Kawai 1 &
- Alistair Griffiths 6
Extreme Physiology & Medicine volume 1 , Article number: 4 ( 2012 ) Cite this article
14k Accesses
2 Citations
29 Altmetric
Metrics details
Photosynthesis maintains aerobic life on Earth, and Joseph Priestly first demonstrated this in his eighteenth-century bell jar experiments using mice and mint plants. In order to demonstrate the fragility of life on Earth, Priestley's experiment was recreated using a human subject placed within a modern-day bell jar.
A single male subject was placed within a sealed, oxygen-depleted enclosure (12.4% oxygen), which contained 274 C 3 and C 4 plants for a total of 48 h. A combination of natural and artificial light was used to ensure continuous photosynthesis during the experiment. Atmospheric gas composition within the enclosure was recorded throughout the study, and physiological responses in the subject were monitored.
After 48 h, the oxygen concentration within the container had risen to 18.1%, and hypoxaemia in the subject was alleviated (arterial oxygen saturation rose from 86% at commencement of the experiment to 99% at its end). The concentration of carbon dioxide rose to a maximum of 0.66% during the experiment.

Conclusions
This simple but unique experiment highlights the importance of plant life within the Earth's ecosystem by demonstrating our dependence upon it to restore and sustain an oxygen concentration that supports aerobic metabolism. Without the presence of plants within the sealed enclosure, the concentration of oxygen would have fallen, and carbon dioxide concentration would have risen to a point at which human life could no longer be supported.
The Earth supports a fragile ecosystem, and its inhabitants depend for their survival upon complex interactions between them, which have developed over billions of years. Imbalance of one component in this bionetwork can have far-reaching effects on organisms whose existence relies upon the presence of other species. Despite the ability to alter their environment in diverse ways, humans are reliant for their survival upon an element derived primarily from plants and produced by chlorophyll during photosynthesis, oxygen (O 2 ).
Photosynthesis is arguably the single most important chemical process on our planet, and the first colour images captured of Earth from space revealed the vast green hues of the landmasses supporting plant life, confirming its dominance within our ecosystem. Using energy from sunlight, chlorophyll strips electrons from water molecules, which then convert atmospheric carbon dioxide (CO 2 ) into carbon compounds, producing O 2 as a byproduct. Whilst mechanisms that use alternative naturally available compounds to release energy exist, the abundance of water on the surface of the Earth meant that photosynthesis rapidly became the foremost bio-energetic pathway on the planet. During the early era of chlorophyll photosynthesis, approximately 2,400 million years ago [ 1 ], the atmosphere was rich in CO 2 , whilst O 2 was scarce. As time progressed and photosynthetic species slowly overwhelmed the surface of the Earth, the concentration of O 2 rose and eventually reached levels we are accustomed to today.
In the early 1770 s, Joseph Priestley conducted a series of experiments that led to the discovery of the intimate relationship between plant and animal life [ 2 ]. In his principal experiment, Priestley placed a mouse within a sealed jar and observed it to eventually perish. When repeated with sprigs of mint within the jar, neither did the animal die ‘nor was it at all inconvenient to a mouse’ [ 2 ]. He had made the breakthrough that plants produce a substance which is life-giving to animals and then went on to describe ‘dephlogisticated air’, which, thanks to the French chemist Antoine Lavoisier, soon became known as ‘oxygen’. The story of photosynthesis was completed in 1779 when a Dutchman, Jan Ingenhousz, demonstrated that the process by which plants produce O 2 is dependent upon light.
We hypothesised that a human could survive within a sealed modern-day bell jar, even if the O 2 concentration within was significantly reduced from the outset, provided that it contained sufficient plant matter to generate O 2 and remove CO 2 via photosynthesis.
Formal ethical approval was not sought for this experiment as it was designed for the purpose of a television demonstration; consent was implied through the subject's involvement in the project and participation in the event. The Chair of the University College London Committee on the Ethics of Non-NHS Human Research approved this strategy. Prior to commencing the experiment, a full medical screening questionnaire was completed by the subject, and he was assessed by a physician with experience in high altitude and acute hypoxia research (DM). The protocol was explained to the subject in full, along with a description of the potential risks and safety measures in place. A standard resuscitation kit was available throughout the experiment, along with bottled supplemental oxygen. A physician trained in Advanced Life Support was also present outside the container throughout the experiment, with the ability to enter the container at any point should there be concerns regarding the welfare of the subject.
We constructed the first human recreation of Priestley's ‘mouse in a bell jar’ experiment to demonstrate the ability of plants to generate sufficient O 2 to sustain human life in an enclosed environment [ 3 ]. A healthy 47-year-old male was placed within a transparent airtight container measuring 2.0 × 2.5 × 6.0 m (30 m 3 , Figure 1 ), itself placed within the rainforest biome at the Eden Project, Cornwall, UK. A selection of plants known for their high photosynthetic yield (under certain environmental conditions) was placed within the container. Prior to the experiment, containerised plants were grown in a peat-free Eden Project Melcourt mix within a standard glasshouse at a relative humidity of 70% to 80% and temperature range of 15°C to 30°C. During this growing phase, the plants were watered with liquid nutrient feed at 20 ml/L (N 177 ppm, P 35 ppm, K 119 ppm, Ca 49 ppm, Mg 17 ppm, B 0.2 ppm, Cu 0.08 ppm, Fe 1.44 ppm, Mn 0.48 ppm, Mo 0.04 ppm and Zn 0.64 ppm). In total, 274 plants consisting of 18 different taxa were placed within the container, with 10,967 leaves (excluding Tillandsia usneoides ) and a total leaf area of 1,106,033 cm 2 (Table 1 ). A mixture of C 3 (ribulose diphosphate carboxylase utilising) and C 4 (phosphoenolpyruvate carboxylase utilising) plants were selected in order to maximise photosynthetic potential within the container. Several C 4 carbon fixation plants were grown, including Miscanthus x giganteus and Zea mays (maize), because they have advantages over C 3 plants, resulting in superior carbon-gaining capacities and photosynthetic efficiency [ 4 ]. During the experiment, the subject regularly irrigated the plants when deemed necessary from a water source within the container.

The sealed container with plants, the subject and external artificial lighting.
In order to more clearly demonstrate oxygen production and highlight the effectiveness of photosynthesis in preserving human life, the environment within the container was rendered hypoxic at the start of the experiment. Three hypoxic generators (Hypoxico Everest Summit II, Hypoxico Inc, New York, NY, USA) were used to reduce the concentration of O 2 in the container. These devices consist of a molecular sieve system that uses zeolite to separate nitrogen from O 2 in the air and consequently provides a nitrogen-rich gas mixture to purge the atmosphere within the container. Connected to the container, and in conjunction with a one-way pressure relief valve, the hypoxic generators reduced the concentration of O 2 to 12.4% prior to commencing the experiment. Once the subject was sealed inside the container and safety procedures had been confirmed, the hypoxic generators were switched off and the one-way valves were closed. Artificial lighting (8 × 2,000 W systems; ARRI, Munich, Germany) was placed around the container externally and switched on at the beginning of the experiment. A split air-conditioning unit (Clima 16 HP Portable Air Conditioner, Toshiba, Tokyo, Japan) was used to maintain temperatures for optimal plant growth and comfort for the subject whilst ensuring a sealed atmosphere. The concentrations of O 2 and CO 2 within the container were monitored with a gas analyser (Aspida, Analox, London, UK) and plotted every hour along with temperature and humidity from a digital hygro-thermometer (Brannan, Cumbria, UK). The subject's heart rate and arterial O 2 saturation (SpO 2 ) were monitored continuously (Johnson and Johnson Dinamap MPS Monitor and Onyx 9500, Nonin, Plymouth, MN, USA); respiratory rate was recorded hourly by manual calculation.
The concentration of O 2 in the container rose throughout the experiment, peaking at 18.1% in the final hour (hour 48; Figure 2 ). The CO 2 concentration fluctuated depending on the subject's activity within the container (declining noticeably during sleep), but there was an overall rise that peaked at 0.66%, approximately halfway through the experiment (Figure 3 ). There was a diurnal variation in temperature (25.3°C to 28.4°C), and humidity varied between 57% and 87%. On entering the hypoxic container, the subject had a heart rate of 90 beats per minute, respiratory rate of 20 breaths per minute and SpO 2 of 86%. These figures returned to the subject's resting normal values as the concentration of O 2 rose within the container. The subject's final SpO 2 was 99% (Figure 4 ).
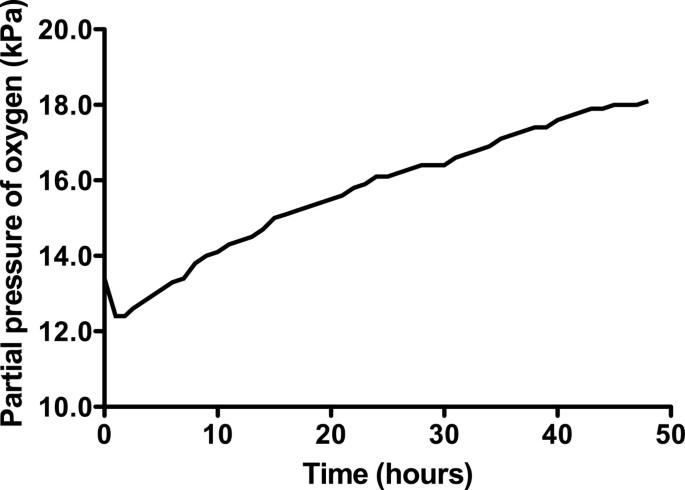
Change in oxygen concentration within the container over time.
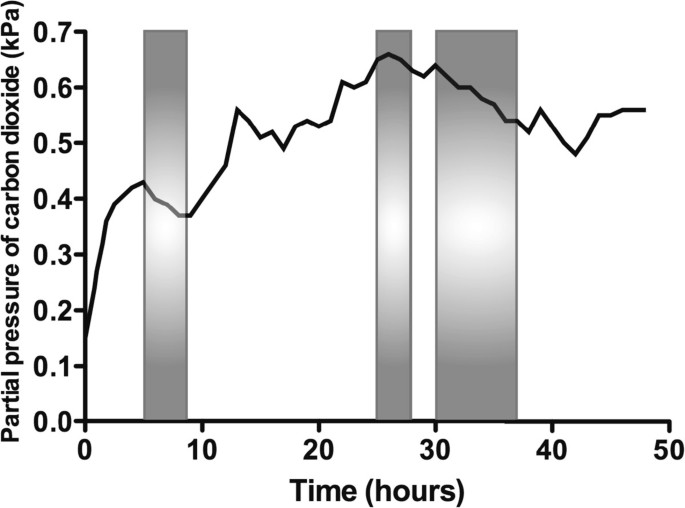
Change in carbon dioxide concentration within the container over time. The shaded areas are those during which the subject was sleeping.
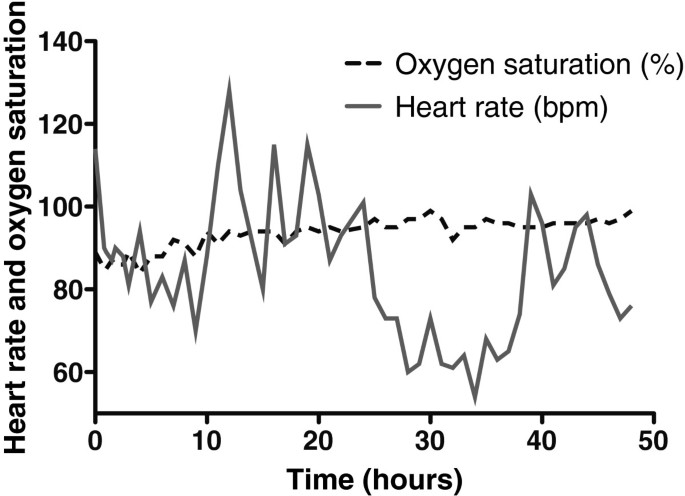
Changes in the subject's oxygen saturation and heart rate during enclosure within the container.
The design of the biological ecosystem in this study was such that human life was sustained for 48 h and the initial hypoxic environment restored to one of near-normal O 2 concentration. In the early 1990's, the ‘Biosphere 2’ experiment was conducted to explore the feasibility of self-sustaining biospheres in space. This grand design consisted of a 200 m 3 atmosphere within a dome that contained eight volunteers, which was designed to sustain them for 2 years [ 5 ]. However, the O 2 concentration within the biosphere dropped from 20.9% to 14.2% after 16 months, so additional O 2 had to be added to the atmosphere [ 6 ]. This decline was traced to a two-step process: firstly, there was O 2 loss to organic soil matter producing CO 2 , and secondly, the CO 2 was being captured by structural concrete to form calcium carbonate [ 5 ]. In the current experiment, the initial O 2 concentration of 12.4% (equivalent to approximately 4,500 m above sea level) resulted in a marked reduction in the subject's SpO 2 and represents an acute hypoxic exposure that is frequently associated with symptoms of altitude-related illness [ 7 ]. During the last few hours of the study, there was a small reduction in rate of the O 2 concentration rise, perhaps due to deterioration in the condition of the plants, noticeable towards the end of the experiment. Direct heating and excessive light exposure, arguably both present in this experiment, can lead to the denaturing of enzymes within chlorophyll [ 8 ]. There were fluctuations in CO 2 concentration throughout the study, with a tendency for it to rise as time progressed (Figure 3 ).
As well as providing an insight into the use of plants to maintain a self-sufficient biosphere, such as would be required on the surface of extra-terrestrial bodies without an atmosphere, our experiment highlights the detrimental effects of a markedly increased CO 2 concentration. CO 2 concentrations have altered dramatically over the course of the Earth's history [ 9 ], and there is much concern that levels are now rising at an alarming rate [ 10 ]. Under certain environmental conditions, increasing the ambient concentration of CO 2 can be beneficial, increasing photosynthetic activity, plant growth and yield [ 11 , 12 ]. Using CO 2 enrichment to increase plant growth and yield is now commonplace in commercial glasshouse crop production, with optimal levels being between 700 and 1,000 ppm [ 13 ]. However, in some species, super-elevated CO 2 concentrations (over 2,000 ppm) induces foliar symptoms of chlorosis and necrosis [ 14 , 15 ], and levels above 10,000 ppm are known to cause damage to young maize plants after 48 h in the form of ‘yellow streaks’ [ 16 ]. During this experiment, the CO 2 levels remained above 2,000 ppm and reached a maximum of 6,600 ppm, yet yellow streaks were observed on the maize plants by the end. It is possible that damage to the maize may have also reduced the photosynthetic yield and the production of O 2 towards the end of this experiment. This study, therefore, provides an insight into the use of plants to maintain a self-sufficient biosphere, such as would be required on the surface of extra-terrestrial bodies without an atmosphere, and the potentially detrimental effects of a dramatically increased CO 2 concentration.
This simple experiment is a humble reminder of the integral relationship between animal and plant life on Earth, in which the former owe their existence to the latter. Without the presence of plants within the sealed environment, the concentration of O 2 would have fallen and CO 2 concentration would have risen to a point at which human life could no longer be supported. Whilst O 2 sustains human life and plants maintain its level within the atmosphere with remarkable efficiency, the fundamental role of photosynthesis is arguably taken for granted. Deprived of plants, the subject within the container would have succumbed to the effects of severe hypoxaemia. The experiment reminds us of our total dependency upon plants, and the ecosystem in which they exist.
Blankenship RE: Origin and early evolution of photosynthesis. Photosynth Res. 1992, 33: 91-111. 10.1007/BF00039173.
Article CAS Google Scholar
Priestley J: Experiments and Observations on Different Kinds of Air. 1774, W. Bowyer and J. Nichols, London
Google Scholar
,: Water the plant, take a breath. Science. 2011, 333: 1685-
Sage RF, Zhu XG: Exploiting the engine of C(4) photosynthesis. J Exp Bot. 2011, 62: 2989-3000. 10.1093/jxb/err179.
Article CAS PubMed Google Scholar
Allen J, Nelson M: Overview and design biospherics and biosphere 2, mission one (1991–1993). Ecol Engin. 1999, 13: 15-29. 10.1016/S0925-8574(98)00089-5.
Article Google Scholar
Dempster WF: Biosphere 2. System dynamics and observations during the initial two-year closure trial. 23rd International conference on environmental systems: July 1993; Colorado. 1993, SAE International, Warrendale
Hackett PH, Roach RC: High-altitude illness. N Engl J Med. 2001, 345: 107-114. 10.1056/NEJM200107123450206.
Bohning RH: Time course of photosyntesis in apple leaves exposed to continuous illumination. Plant Physiol. 1949, 24: 222-240. 10.1104/pp.24.2.222.
Article PubMed Central CAS PubMed Google Scholar
Pearson PN, Palmer MR: Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000, 406: 695-699. 10.1038/35021000.
Alley R, Berntsen T, Bindoff NL, Chen Z, Chidthaisong AF P, Gregory J, Hegel G, Heimann M, Hewitson B: Climate Change 2007: The Physical Science Basis, Summary for Policymakers. 2007, Intergovernmental Panel on Climate Change Secretariat, Geneva
Körner C: Biosphere responses to CO2 enrichment. Ecol Appl. 2000, 10: 1590-1619.
Drake BG, Gonzalez-Meler MA, Long SP: More efficient plants: a consequence of rising atmospheric CO2?. Annu Rev Plant Physiol Plant Mol Biol. 1997, 48: 609-639. 10.1146/annurev.arplant.48.1.609.
Hanan JJ: Greenhouses. Advanced technology for protected horticulture. 1998, CRC Press, London
Ehret DL, Jolliffe PA: Leaf injury to bean plant grown in carbon dioxide enriched atmospheres. Can J Bot. 1985, 63: 2015-2020.
CAS Google Scholar
van Berkel N: Injurious effects of high CO2 concentration on cucumber, tomato, chrysanthemum and gerbera. Acta Horticulturae. 1984, 162: 101-112.
Schwarz M: Carbon toxicity in plants. Acta Horticulturae. 1999, 481: 685-688.
Download references
Acknowledgements
We would like to thank the BBC Scotland team for the ‘How to Make a Planet’ series and all the staff at the Eden Project in Cornwall who made this experiment possible.
Author information
Authors and affiliations.
UCL Centre for Altitude, Space and Extreme Environment Medicine, Portex Unit, Institute of Child Health, 30 Guilford Street, London, WC1N 1EH, UK
Daniel Martin, Edward Gilbert & Grace Kawai
Division of Surgery and Interventional Science, University College London, 9th Floor, Royal Free Hospital, London, NW3 2QG, UK
Daniel Martin
BBC Television, Zone 2.20, BBC Pacific Quay, Glasgow, G51 1DA, UK
Andrew Thompson
School of Geography, Earth & Environmental Sciences, Plymouth University, Plymouth, PL4 8AA, UK
Iain Stewart
Centre of Human & Aerospace Physiological Sciences, School of Biomedical Sciences, King's College London, London, SE1 1UL, UK
Katrina Hope
The Eden Project, Bodelva, Cornwall, PL24 2SG, UK
Alistair Griffiths
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Daniel Martin .
Additional information
Competing interests.
The author declares that they have no competing interests.
Authors’ contributions
AT conceived the idea, and the experiment was designed by AT, AG and DM. The experiment was conducted by DM, KH, EG, GK, AT and IS. Data were analysed by DM, and the manuscript was written by DM, AG and EG. All authors discussed the results and implications and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, authors’ original file for figure 4, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Martin, D., Thompson, A., Stewart, I. et al. A paradigm of fragile Earth in Priestley's bell jar. Extrem Physiol Med 1 , 4 (2012). https://doi.org/10.1186/2046-7648-1-4
Download citation
Received : 14 March 2012
Accepted : 04 September 2012
Published : 04 September 2012
DOI : https://doi.org/10.1186/2046-7648-1-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Carbon dioxide
- Photosynthesis
Extreme Physiology & Medicine
ISSN: 2046-7648
- General enquiries: [email protected]
- Women's History
- Latino History
- Collections
Joseph Priestley (1733–1804) used this bell jar in his Northumberland, Pennsylvania laboratory. Priestley, the noted chemist whose accomplishments include the discovery of oxygen, was born in England. He lived and worked in Birmingham for many years, but his views as a Dissenter and an advocate of the French Revolution incited an angry mob into burning down his house and laboratory. In 1794 he fled to America, eventually settling in Northumberland, near Philadelphia. His great-great-granddaughter, Frances Priestley, donated his surviving laboratory ware to the Smithsonian in 1883.
The transparent glass bell jar provided a useful shape for trapping and observing gases. A chemical sample could be suspended in the jar and ignited by passing a beam of focused light or heat through the glass. Any gases emitted from its burning would be collected for further study.
Glassmaker William Parker of 69 Fleet St., London or his son Samuel likely made this bell jar. The Parkers supplied Priestley with laboratory glassware free of charge, even after his move to the United States from London. Priestley wrote in a letter to Rev. Samuel Palmer, of his new home in Northumberland, Pennsylvania: “I have more advantages [in respect to experiments] than you could easily imagine in this remote place. I want hardly anything but a glass house.” Indeed, without a local supplier, getting glassware to Northumberland was quite a challenge. A letter to Samuel Parker dated January 20, 1795 details Priestley’s plan to have his most recent shipment brought from Philadelphia to Northumberland via a sleigh, “which is our best method of conveyance in winter.”
Badash, Lawrence. 1964. “Joseph Priestley’s Apparatus for Pneumatic Chemistry.” Journal of the History of Medicine and Allied Sciences XIX (2): 139–55. doi:10.1093/jhmas/XIX.2.139.
National Museum of American History Accession File #13305
Priestley, Joseph, and John Towill Rutt. 1817. The Theological and Miscellaneous Works of Joseph Priestley. Vol. I Part 2. [London : Printed by G. Smallfield. http://archive.org/details/theologicalmisce0102prie.
Used By: Priestley, Joseph
Location: Currently not on view
Subject: Science & Scientific Instruments Chemistry
See more items in: Medicine and Science: Chemistry , Joseph Priestley , Science & Mathematics
Exhibition:
Exhibition Location:
Credit Line: Gift of Miss Frances D. Priestley
Data Source: National Museum of American History
Id Number: CH.315345 Accession Number: 13305 Catalog Number: 315345
Object Name: bell jar
Measurements: overall: 20 in x 7 1/2 in; 50.8 cm x 19.05 cm
Metadata Usage: CC0
Guid: https://n2t.net/ark:/65665/ng49ca746a0-e68a-704b-e053-15f76fa0b4fa
Record Id: nmah_1764
Our collection database is a work in progress. We may update this record based on further research and review. Learn more about our approach to sharing our collection online .
If you would like to know how you can use content on this page, see the Smithsonian's Terms of Use . If you need to request an image for publication or other use, please visit Rights and Reproductions .
Talk to our experts
1800-120-456-456
Early Experiments on Photosynthesis

Photosynthesis is a process by which plants produce their food. It is a photochemical process in which the light energy is absorbed by the plants and is converted into chemical energy to produce oxygen. This process was followed by the plants for ages. But it’s discovery and identification were done only in 1800 and several scientists conducted many different types of experiments to prove the existence of photosynthesis.
Photosynthesis Discovery – Early Experiments
The process of photosynthesis is carried by some of the required raw materials like water, carbon dioxide, and cellular components like plastids. Plants make use of these raw materials to synthesize carbohydrates in the presence of sunlight. These key features of photosynthesis were revealed during the mid-nineteenth century.
Some of the experiments that were conducted by the early scientists to explore photosynthesis in a better way are -
Experiment to Prove the Importance of Carbon Dioxide in Photosynthesis
Materials Required: A healthy potted plant, a wide-mouthed glass bottle with a split cork, potassium hydroxide solution (KOH), and starch solution.
Experiment:
Take a healthy potted plant and keep it in the darkroom for two to three days to ensure leaves are free from starch.
In a wide-mouthed glass bottle add 10-15 ml of potassium hydroxide solution and split the cork vertically.
Now minutely, insert half part of a leaf into a glass bottle through the split cork and the other half exposed to air.
Place the complete unit undisturbed in sunlight for about 3 – 4 hours.
Remove the leaf after 4 hours from the plant and slowly remove it out from the bottle and test it with the starch solution.
We can observe that the half part leaf which was inside the glass bottle (KOH solution) did not show any colour change but the other half part exposed to surroundings became dark brown indicating the presence of starch in it.
Conclusion: In this experiment, we can conclude that carbon dioxide is essential for photosynthesis. Both portions of leaf received the same amount of water, chloroplasts, and sunlight but the half part which was inside the glass bottle did not receive carbon dioxide.
Later, many improvised experiments were conducted by scientists to analyze the essential components for photosynthesis. Joseph Priestley (1733-1804) was the first scientist amongst others to carry out these experiments.
Experiment by Joseph Priestley
After conducting a series of experiments in 1770, Joseph Priestley concluded that the essentiality of air for photosynthesis and also for the growth of plants.
Materials Required: A candle, rat, a bell jar, and a plant.
Firstly, a burning candle and a rat were kept together in the single bell jar.
After some time, the candle extinguished and the rat died.
For the second time, he kept a burning candle, rat, and a green plant all together in the bell jar.
He observed that neither the candle got extinguished, nor did the rat die.
Conclusion: Based on his observations, the scientist Priestley concluded that in the first case, the air in the bell jar got polluted by the candle and the existence of the rat. However, in the second case, the plant restored the air that was spoiled by the candle and the rat. But this function of the plants was not revealed quite soon by scientists.
Other Experiments
Scientist Jan Ingenhousz also conducted experiments using the same set-up but the twist was the presence of sunlight that was highlighted as being an essential product for plants to refresh the air that was polluted by the candle or rat.
Jean Senebier came to a conclusion which said that plants absorb carbon dioxide and release oxygen during photosynthesis.
Julius Robert Mayer demonstrated that plants convert light energy into chemical energy.
Later, Julius von Sachs revealed that glucose was produced by plants.
T.W Engelmann discovered the role of chlorophylls and Cornelius van Niel uncovered that the release of oxygen by plants is from water (H 2 O), not from carbon dioxide. He also gave the general photosynthesis equation.
An outline was drawn for the process of photosynthesis by scientists. They concluded that light is essential for photosynthesis, and plants use carbon dioxide and water for the preparation of glucose (carbohydrate), where water molecules are the hydrogen donors and oxygen (O 2 ) is the by-product of this biological process.
FAQs on Early Experiments on Photosynthesis
1. Define Photosynthesis
Photosynthesis is a process by which plants produce their food. It is a photochemical process in which the light energy is absorbed by the plants and is converted into chemical energy to produce oxygen. This process was followed by the plants for ages. But it’s discovery and identification were done only in 1800 and several scientists conducted many different types of experiments to prove the existence of photosynthesis.
2. What Were the Materials Used for the Experiment of Photosynthesis?
The materials used for the experiment of Photosynthesis was -
A healthy potted plant
A wide-mouthed glass bottle with a split cork
Potassium hydroxide solution (KOH)
Starch solution
Biology • Class 11

IMAGES
COMMENTS
En español: Joseph Priestley y el descubrimiento del oxígeno. Landmark Lesson Plan: Joseph Priestley, Discoverer of Oxygen. When Joseph Priestley discovered oxygen in 1774, he answered age-old questions of why and how things burn. An Englishman by birth, Priestley was deeply involved in politics and religion, as well as science.
Oct 9, 2017 · Joseph Priestly was one of the first scientists to perform these experiments. Experiment by Joseph Priestley. In 1770, after a series of experiments, Joseph Priestley came to a conclusion regarding the essentiality of air for photosynthesis and also for the growth of plants. Materials required: A bell jar, candle, rat, and a plant. Experiment:
Joseph Priestly's bell jar experiment shows that plants produce oxygen. In 1771, Joseph Priestley conducted an experiment where he placed a sprig of mint inside a bell jar and sealed it. He then placed the jar in direct sunlight. Over time, Priestley observed that the mint sprig released a gas that kept a mouse alive when placed in the jar.
Photosynthesis maintains aerobic life on Earth, and Joseph Priestly first demonstrated this in his eighteenth-century bell jar experiments using mice and mint plants. In order to demonstrate the fragility of life on Earth, Priestley's experiment was recreated using a human subject placed within a modern-day bell jar. Methods
In 1771 and 1772, Joseph Priestley performed a series of experiments that revealed the essential role of air in the growth of green plants. Priestley observed that a candle burning in a closed space such as a bell jar, soon gets extinguished. Similarly, a mouse would soon suffocate in a closed space.
Sep 4, 2012 · Background Photosynthesis maintains aerobic life on Earth, and Joseph Priestly first demonstrated this in his eighteenth-century bell jar experiments using mice and mint plants. In order to demonstrate the fragility of life on Earth, Priestley's experiment was recreated using a human subject placed within a modern-day bell jar. Methods A single male subject was placed within a sealed, oxygen ...
Sep 4, 2012 · Earth, Priestley's experiment was recreated using a human subject place d within a modern-day bell jar. Methods: A single male subject was placed withi n a sea led, oxygen-depleted enclosure (12.4 ...
His great-great-granddaughter, Frances Priestley, donated his surviving laboratory ware to the Smithsonian in 1883. Description (Brief) The transparent glass bell jar provided a useful shape for trapping and observing gases. A chemical sample could be suspended in the jar and ignited by passing a beam of focused light or heat through the glass.
Joseph Priestley (1733-1804) was the first scientist amongst others to carry out these experiments. Experiment by Joseph Priestley. After conducting a series of experiments in 1770, Joseph Priestley concluded that the essentiality of air for photosynthesis and also for the growth of plants. Materials Required: A candle, rat, a bell jar, and a ...
• Bell Jar • L.A.W.N. – light, air, water, and soil • A rubric to record experiment results The first plant should be placed in a closet or other dark location, where it should receive air and water, and be rooted in soil. In England in 1773, Joseph Priestley put a mint plant in a bell jar to prove his