
- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Miller-Urey Experiment
The Miller-Urey Experiment was a landmark experiment to investigate the chemical conditions that might have led to the origin of life on Earth. The scientist Stanley Miller, under the supervision of the Nobel laureate scientist Harold Urey conducted it in 1952 at the University of Chicago. They tried to recreate the conditions that could have existed in the first billion years of the Earth’s existence (also known as the Early Earth) to check the said chemical transformations.
Miller-Urey Experiment And The Primordial Soup Theory
The experiment tested the primordial or primeval soup theory developed independently by the Soviet biologist A.I. Oparin and English scientist J.B.S. Haldane in 1924 and 1929 respectively. The theory propounds the idea that the complex chemical components of life on Earth originated from simple molecules occurring naturally in the reducing atmosphere of the Early Earth, sans oxygen. Lightning and rain energized the said atmosphere to create simple organic compounds that formed an organic “soup”. The so-called soup underwent further changes giving rise to more complex organic polymers and finally life.
The Miller-Urey Experiment In Support Of Abiogenesis
From what was explained in the previous paragraph, it can undoubtedly be considered as a classic experiment to demonstrate abiogenesis. For those who are not conversant with the term, abiogenesis is the process responsible for the development of living beings from non-living or abiotic matter. It is thought to have taken place on the Earth about 3.8 to 4 billion years ago.
Miller-Urey Experiment Apparatus and Procedure
The groundbreaking experiment used a sterile glass flask of 5 liters attached with a pair of electrodes, to hold water (H 2 O), methane (CH 4 ), ammonia (NH 3 ) and hydrogen (H 2 ), the major components of primitive Earth. This was connected to another glass flask of 500 ml capacity half filled with water. On heating it, the water vaporized to fill the larger container with water vapor. The electrodes induced continuous electrical sparks in the gas mixture to simulate lightning. When the gas was cooled, the condensed water made its way into a U-shaped trap at the base of the apparatus.
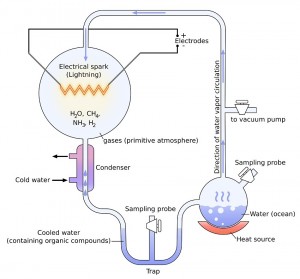
After electrical sparking had continued for a day, the solution in the trap turned pink in color. At the end of a week, the boiling flask was removed, and mercuric chloride added to prevent microbial contamination. After stopping the chemical reaction, the scientist duo examined the cooled water collected to find that 10-15% of the carbon present in the system was in the form of organic compounds. 2% of carbon went into the formation of various amino acids, including 13 of the 22 amino acids essential to make proteins in living cells, glycine being the most abundant.
Though the result was the production of only simple organic molecules and not a complete living biochemical system, still the simple prebiotic experiment could, to a considerable extent, prove the primordial soup hypothesis.
Miller-Urey Experiment Animation
Chemistry of the miller and urey experiment.
The components of the mixture can react among themselves to produce formaldehyde (CH 2 O), hydrogen cyanide (HCN) and other intermediate compounds.
CO 2 → CO + [O] (atomic oxygen)
CH 4 + 2[O] → CH 2 O + H 2 O
CO + NH 3 → HCN + H 2 O
CH 4 + NH 3 → HCN + 3H 2
The ammonia, formaldehyde and HCN so produced react by a process known as Strecker synthesis to form biomolecules including amino acids.
CH 2 O + HCN + NH 3 → NH 2 -CH 2 -CN + H 2 O
NH 2 -CH 2 -CN + 2H 2 O → NH 3 + NH 2 -CH 2 -COOH (glycine)
In addition to the above, formaldehyde and water can react by Butlerov’s reaction to produce a variety of sugars like ribose, etc.
Though later studies have indicated that the reducing atmosphere as replicated by Miller and Urey could not have prevailed on primitive Earth, still, the experiment remains to be a milestone in synthesizing the building blocks of life under abiotic conditions and not from living beings themselves.
https://www.bbc.co.uk/bitesize/guides/z2gjtv4/revision/1
https://www.juliantrubin.com/bigten/miller_urey_experiment.html
Article was last reviewed on Thursday, February 2, 2023
Related articles

One response to “Miller-Urey Experiment”
This experiment is currently seen as not sufficient to support abiogenesis. See Stephen C. Meyer, James Tour.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
The Science Notes
Your Best Science Education Portal
Miller Urey Experiment: Hypothesis, Steps, Conclusions, and Limitations
The Miller Urey Experiment played a crucial role in investigating the origin of life on our planet. This comprehensive guide explores the experiment’s hypothesis, step-by-step process, key findings, and limitations, shedding light on its significance in unraveling the mysteries of life’s beginnings.
Oparin-Haldane Hypothesis
The Oparin-Haldane Hypothesis, proposed by Aleksandr Oparin and J.B.S. Haldane, postulates that life didn’t spontaneously emerge on early Earth due to different environmental conditions. It suggests that life gradually evolved from chemical reactions, starting with the combination of atoms into inorganic molecules and the subsequent formation of simple organic compounds. These compounds then assembled into complex organic structures, ultimately leading to the emergence of the first cell.
Steps of the Miller Urey Experiment
The Miller-Urey experiment, conducted in 1953 by Stanley L. Miller and Harold C. Urey, aimed to simulate early Earth’s conditions and test the Oparin-Haldane Hypothesis. Here are the key steps of the experiment:
Simulating Early Earth’s Atmosphere: The researchers recreated early Earth’s atmosphere in a closed system using a mixture of gases believed to be present during that era. They used a mixture of gases, including methane (CH4), ammonia (NH3), water vapor (H2O), and hydrogen (H2).
Introduction of Energy: Sparks or electric discharges were introduced to simulate the energy sources on early Earth, such as lightning strikes.
Circulation and Condensation: The gas mixture and energy were circulated continuously, mimicking Earth’s water cycle and allowing for the formation of various organic compounds.
Collection and Analysis: Samples were collected from the closed system and analyzed using chromatography and spectrometry to identify and characterize the organic compounds formed during the experiment.
Results and Findings: The experiment produced a variety of organic molecules, including amino acids—the building blocks of proteins —supporting the notion that early Earth’s conditions could have facilitated the synthesis of organic compounds essential for life’s origin.
Conclusions of the Miller Urey Experiment
The Miller-Urey experiment yielded significant conclusions, including:
- Organic compounds, including amino acids, can be synthesized from inorganic materials under simulated early Earth conditions.
- Basic building blocks of life may have emerged spontaneously from non-living matter.
- The experiment demonstrated the potential for diverse organic compound formation, including rare amino acids.
- External energy sources played a crucial role in facilitating chemical reactions and organic compound synthesis.
- The experiment offered insights into the chemical reactions that might have occurred in early Earth’s atmosphere.
- The findings supported the concept of abiogenesis, where life can arise from non-living matter through natural processes.
- The Miller-Urey experiment laid the foundation for further research in prebiotic chemistry and the study of life’s origins.
Limitations of the Miller Urey Experiment
It’s important to consider the limitations of the Miller-Urey experiment, which include:
- The experiment’s simulation of early Earth’s atmosphere may not perfectly represent the actual conditions.
- The specific gases used may not accurately reflect the true composition of early Earth’s atmosphere.
- The experiment’s short duration and scale may not fully capture the complexity and length of natural processes involved in life’s origin.
- While the experiment produced organic compounds, it didn’t address the assembly of complex biomolecules or replicating systems crucial for life’s origin.
Ongoing Debates and Significance
Critics argue that the experiment oversimplifies the interconnected nature of biochemical systems and may not fully represent the processes behind life’s origin. There is an ongoing debate regarding the specific conditions and pathways leading to life’s emergence, with the Miller-Urey experiment presenting one plausible scenario. While it doesn’t address the origin of genetic information or self-replicating systems, subsequent research has refined and expanded upon its findings, leading to revised interpretations. The Miller-Urey experiment remains a significant milestone in our understanding of prebiotic chemistry and contributes to unraveling the complex puzzle of life’s origin.
In conclusion, the Miller-Urey experiment’s hypothesis, steps, conclusions, and limitations provide valuable insights into the origin of life on Earth. It serves as a foundation for further research, stimulating ongoing debates and refining our understanding of life’s emergence from non-living matter.
Learn more:
Amino Acids: Types, Functions, Sources, and Differences between Essential and Non-Essential Amino Acids
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- Biology Article
Miller Urey Experiment
Miller and urey experiment.
Stanley L. Muller and Harold C. Urey performed an experiment to describe the origin of life on earth. They were of the idea that the early earth’s atmosphere was able to produce amino acids from inorganic matter. The two biologists made use of methane, water, hydrogen, and ammonia which they considered were found in the early earth’s atmosphere. The chemicals were sealed inside sterile glass tubes and flasks connected together in a loop and circulated inside the apparatus.
One flask is half-filled with water and the other flask contains a pair of electrodes. The water vapour was heated and the vapour released was added to the chemical mixture. The released gases circulated around the apparatus imitating the earth’s atmosphere. The water in the flask represents the water on the earth’s surface and the water vapour is just like the water evaporating from lakes, and seas. The electrodes were used to spark the fire to imitate lightning and storm through water vapour.
The vapours were cooled and the water condensed. This condensed water trickles back into the first water flask in a continuous cycle. Miller and Urey examined the cooled water after a week and observed that 10-15% of the carbon was in the form of organic compounds. 2% of carbon had formed 13 amino acids . Yet, the Miller and Urey experiments were condemned by their fellow scientists.
Also read: Origin Of Life
Criticism of the Miller Urey Experiment
The experiment failed to explain how proteins were responsible for the formation of amino acids. A few scientists have contradicted that the gases used by Miller and Urey are not as abundant as shown in the experiment. They were of the notion that the gases released by the volcanic eruptions such as oxygen, nitrogen, and carbon dioxide make up the atmosphere. Therefore, the results are not reliable.

Oparin and Haldane
In the early 20th century, Oparin and Haldane suggested that if the atmosphere of the primitive earth was reducing and if it had sufficient supply of energy such as ultraviolet radiations and lightning, organic compounds would be synthesized at a wide range.
Oparin believed that the organic compounds would have undergone a series of reactions to form complex molecules. He suggested that the molecules formed coacervates in the aqueous environment.
Haldane proposed that the atmosphere of the primordial sea was devoid of oxygen, and was a composed of ammonia, carbon dioxide, and ultraviolet light. This gave rise to a host of organic compounds. The sea contained large amounts of organic monomers and polymers, and the sea was called a ‘hot dilute soup’. He conceived that the polymers and monomers acquired lipid membranes. The molecules further developed and gave rise to the first living organism. ‘Prebiotic soup’ was the term coined by Haldane.
Also read: Evolution of Life on Earth
For more information on the Miller Urey Experiment, visit BYJU’S Biology website, or go to BYJU’S app.
Further Reading:
- Genetic Drift
- Vestigial Organs
- Biogenetic Law
- Biosafety Issues
- Mass Extinctions

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Icon 1 — The Miller-Urey Experiment
The experiment itself
The understanding of the origin of life was largely speculative until the 1920s, when Oparin and Haldane, working independently, proposed a theoretical model for "chemical evolution." The Oparin-Haldane model suggested that under the strongly reducing conditions theorized to have been present in the atmosphere of the early earth (between 4.0 and 3.5 billion years ago), inorganic molecules would spontaneously form organic molecules (simple sugars and amino acids). In 1953, Stanley Miller, along with his graduate advisor Harold Urey, tested this hypothesis by constructing an apparatus that simulated the Oparin-Haldane "early earth." When a gas mixture based on predictions of the early atmosphere was heated and given an electrical charge, organic compounds were formed ( Miller, 1953 ; Miller and Urey, 1959 ). Thus, the Miller-Urey experiment demonstrated how some biological molecules, such as simple amino acids, could have arisen abiotically, that is through non-biological processes, under conditions thought to be similar to those of the early earth. This experiment provided the structure for later research into the origin of life. Despite many revisions and additions, the Oparin-Haldane scenario remains part of the model in use today. The Miller-Urey experiment is simply a part of the experimental program produced by this paradigm.
Wells boils off
Wells says that the Miller-Urey experiment should not be taught because the experiment used an atmospheric composition that is now known to be incorrect. Wells contends that textbooks don't discuss how the early atmosphere was probably different from the atmosphere hypothesized in the original experiment. Wells then claims that the actual atmosphere of the early earth makes the Miller-Urey type of chemical synthesis impossible, and asserts that the experiment does not work when an updated atmosphere is used. Therefore, textbooks should either discuss the experiment as an historically interesting yet flawed exercise or not discuss it at all. Wells concludes by saying that textbooks should replace their discussions of the Miller-Urey experiment with an "extensive discussion" of all the problems facing research into the origin of life.
These allegations might seem serious; however, Wells's knowledge of prebiotic chemistry is seriously flawed. First, Wells's claim that researchers are ignoring the new atmospheric data, and that experiments like the Miller-Urey experiment fail when the atmospheric composition reflects current theories, is simply false. The current literature shows that scientists working on the origin and early evolution of life are well aware of the current theories of the earth's early atmosphere and have found that the revisions have little effect on the results of various experiments in biochemical synthesis. Despite Wells's claims to the contrary, new experiments since the Miller-Urey ones have achieved similar results using various corrected atmospheric compositions ( Figure 1 ; Rode, 1999 ; Hanic et al., 2000 ). Further, although some authors have argued that electrical energy might not have efficiently produced organic molecules in the earth's early atmosphere, other energy sources such as cosmic radiation (e.g., Kobayashi et al., 1998 ), high temperature impact events (e.g., Miyakawa et al., 2000 ), and even the action of waves on a beach ( Commeyras, et al., 2002 ) would have been quite effective.
Even if Wells had been correct about the Miller-Urey experiment, he does not explain that our theories about the origin of organic "building blocks" do not depend on that experiment alone ( Orgel, 1998a ). There are other sources for organic "building blocks," such as meteorites, comets, and hydrothermal vents. All of these alternate sources for organic materials and their synthesis are extensively discussed in the literature about the origin of life, a literature that Wells does not acknowledge. In fact, what is most striking about Wells's extensive reference list is the literature that he has left out. Wells does not mention extraterrestrial sources of organic molecules, which have been widely discussed in the literature since 1961 (see Oró, 1961 ; Whittet, 1997 ; Irvine, 1998 ). Wells apparently missed the vast body of literature on organic compounds in comets (e.g. Oró, 1961 ; Anders, 1989 ; Irvine, 1998 ), carbonaceous meteorites (e.g. Kaplan et al., 1963 ; Hayes, 1967 ; Chang, 1994; Maurette, 1998 ; Cooper et al., 2001 ), and conditions conducive to the formation of organic compounds that exist in interstellar dust clouds ( Whittet, 1997 ).
Wells also fails to cite the scientific literature on other terrestrial conditions under which organic compounds could have formed. These non-atmospheric sources include the synthesis of organic compounds in a reducing ocean (e.g., Chang, 1994 ), at hydrothermal vents (e.g., Andersson, 1999 ; Ogata et al., 2000 ), and in volcanic aquifers ( Washington, 2000 ). A cursory review of the literature finds more than 40 papers on terrestrial prebiotic chemical synthesis published since 1997 in the journal Origins of life and the evolution of the biosphere alone. Contrary to Wells's presentation, there appears to be no shortage of potential sources for organic "building blocks" on the early earth.
Instead of discussing this literature, Wells raises a false "controversy" about the low amount of free oxygen in the early atmosphere. Claiming that this precludes the spontaneous origin of life, he concludes that "[d]ogma had taken the place of empirical science" ( Wells 2000 :18). In truth, nearly all researchers who work on the early atmosphere hold that oxygen was essentially absent during the period in which life originated ( Copley, 2001 ) and therefore oxygen could not have played a role in preventing chemical synthesis. This conclusion is based on many sources of data , not "dogma." Sources of data include fluvial uraninite sand deposits ( Rasmussen and Buick, 1999 ) and banded iron formations ( Nunn, 1998 ; Copley, 2001 ), which could not have been deposited under oxidizing conditions. Wells also neglects the data from paleosols (ancient soils) which, because they form at the atmosphere-ground interface, are an excellent source to determine atmospheric composition ( Holland, 1994 ). Reduced paleosols suggest that oxygen levels were very low before 2.1 billion years ago ( Rye and Holland, 1998 ). There are also data from mantle chemistry that suggest that oxygen was essentially absent from the earliest atmosphere ( Kump et al. 2001 ). Wells misrepresents the debate as over whether oxygen levels were 5/100 of 1%, which Wells calls "low," or 45/100 of 1%, which Wells calls "significant." But the controversy is really over why it took so long for oxygen levels to start to rise. Current data show that oxygen levels did not start to rise significantly until nearly 1.5 billion years after life originated ( Rye and Holland, 1998 ; Copley, 2001 ). Wells strategically fails to clarify what he means by "early" when he discusses the amount of oxygen in the "early" atmosphere. In his discussion he cites research about the chemistry of the atmosphere without distinguishing whether the authors are referring to times before, during, or after the period when life is thought to have originated. Nearly all of the papers he cites deal with oxygen levels after 3.0 billion years ago. They are irrelevant, as chemical data suggest that life arose 3.8 billion years ago ( Chang, 1994 ; Orgel, 1998b ), well before there was enough free oxygen in the earth's atmosphere to prevent Miller-Urey-type chemical synthesis.
Finally, the Miller-Urey experiment tells us nothing about the other stages in the origin of life, including the formation of a simple genetic code (PNA or "peptide"-based codes and RNA-based codes) or the origin of cellular membranes (liposomes), some of which are discussed in all the textbooks that Wells reviewed. The Miller-Urey experiment only showed one possible route by which the basic components necessary for the origin of life could have been created, not how life came to be. Other theories have been proposed to bridge the gap between the organic "building blocks" and life. The "liposome" theory deals with the origin of cellular membranes, the RNA-world hypothesis deals with the origin of a simple genetic code, and the PNA (peptide-based genetics) theory proposes an even simpler potential genetic code ( Rode, 1999 ). Wells doesn't really mention any of this except to suggest that the "RNA world" hypothesis was proposed to "rescue" the Miller-Urey experiment. No one familiar with the field or the evidence could make such a fatuous and inaccurate statement. The Miller-Urey experiment is not relevant to the RNA world, because RNA was constructed from organic "building blocks" irrespective of how those compounds came into existence ( Zubay and Mui, 2001 ). The evolution of RNA is a wholly different chapter in the story of the origin of life, one to which the validity of the Miller-Urey experiment is irrelevant.
What the textbooks say
All of the textbooks reviewed contain a section on the Miller-Urey experiment. This is not surprising given the experiment's historic role in the understanding of the origin of life. The experiment is usually discussed over a couple of paragraphs (see Figure 2 ), a small proportion (roughly 20%) of the total discussion of the origin and early evolution of life. Commonly, the first paragraph discusses the Oparin-Haldane scenario, and then a second outlines the Miller-Urey test of that scenario. All textbooks contain either a drawing or a picture of the experimental apparatus and state that it was used to demonstrate that some complex organic molecules (e.g., simple sugars and amino acids, frequently called "building blocks") could have formed spontaneously in the atmosphere of the early earth. Textbooks vary in their descriptions of the atmospheric composition of the early earth. Five books present the strongly reducing atmosphere of the Miller-Urey experiment, whereas the other five mention that the current geochemical evidence points to a slightly reducing atmosphere. All textbooks state that oxygen was essentially absent during the period in which life arose. Four textbooks mention that the experiment has been repeated successfully under updated conditions. Three textbooks also mention the possibility of organic molecules arriving from space or forming at deep-sea hydrothermal vents ( Figure 2 ). No textbook claims that these experiments conclusively show how life originated; and all textbooks state that the results of these experiments are tentative.
It is true that some textbooks do not mention that our knowledge of the composition of the atmosphere has changed. However, this does not mean that textbooks are "misleading" students, because there is more to the origin of life than just the Miller-Urey experiment. Most textbooks already discuss this fact. The textbooks reviewed treat the origin of life with varying levels of detail and length in "Origin of life" or "History of life" chapters. These chapters are from 6 to 24 pages in length. In this relatively short space, it is hard for a textbook, particularly for an introductory class like high school biology, to address all of the details and intricacies of origin-of-life research that Wells seems to demand. Nearly all texts begin their origin of life sections with a brief description of the origin of the universe and the solar system; a couple of books use a discussion of Pasteur and spontaneous generation instead (and one discusses both). Two textbooks discuss how life might be defined. Nearly all textbooks open their discussion of the origin of life with qualifications about how the study of the origin of life is largely hypothetical and that there is much about it that we do not know.
Wells's evaluation
As we will see in his treatment of the other "icons," Wells's criteria for judging textbooks stack the deck against them, ensuring failure. No textbook receives better than a D for this "icon" in Wells's evaluation, and 6 of the 10 receive an F. This is largely a result of the construction of the grading criteria. Under Wells's criteria (Wells 2000:251-252), any textbook containing a picture of the Miller-Urey apparatus could receive no better than a C, unless the caption of the picture explicitly says that the experiment is irrelevant, in which case the book would receive a B. Therefore, the use of a picture is the major deciding factor on which Wells evaluated the books, for it decides the grade irrespective of the information contained in the text! A grade of D is given even if the text explicitly points out that the experiment used an incorrect atmosphere, as long as it shows a picture. Wells pillories Miller and Levine for exactly that, complaining that they bury the correction in the text. This is absurd: almost all textbooks contain pictures of experimental apparatus for any experiment they discuss. It is the text that is important pedagogically, not the pictures. Wells's criteria would require that even the intelligent design "textbook" Of Pandas and People would receive a C for its treatment of the Miller-Urey experiment.
In order to receive an A, a textbook must first omit the picture of the Miller-Urey apparatus (or state explicitly in the caption that it was a failure), discuss the experiment, but then state that it is irrelevant to the origin of life. This type of textbook would be not only scientifically inaccurate but pedagogically deficient.
Why we should still teach Miller-Urey
The Miller-Urey experiment represents one of the research programs spawned by the Oparin-Haldane hypothesis. Even though details of our model for the origin of life have changed, this has not affected the basic scenario of Oparin-Haldane. The first stage in the origin of life was chemical evolution. This involves the formation of organic compounds from inorganic molecules already present in the atmosphere and in the water of the early earth. This spontaneous organization of chemicals was spawned by some external energy source. Lightning (as Oparin and Haldane thought), proton radiation, ultraviolet radiation, and geothermal or impact-generated heat are all possibilities.
The Miller-Urey experiment represents a major advance in the study of the origin of life. In fact, it marks the beginning of experimental research into the origin of life. Before Miller-Urey, the study of the origin of life was merely theoretical. With the advent of "spark experiments" such as Miller conducted, our understanding of the origin of life gained its first experimental program. Therefore, the Miller-Urey experiment is important from an historical perspective alone. Presenting history is good pedagogy because students understand scientific theories better through narratives. The importance of the experiment is more than just historical, however. The apparatus Miller and Urey designed became the basis for many subsequent "spark experiments" and laid a groundwork that is still in use today. Thus it is also a good teaching example because it shows how experimental science works. It teaches students how scientists use experiments to test ideas about prehistoric, unobserved events such as the origin of life. It is also an interesting experiment that is simple enough for most students to grasp. It tested a hypothesis, was reproduced by other researchers, and provided new information that led to the advancement of scientific understanding of the origin of life. This is the kind of "good science" that we want to teach students.
Finally, the Miller-Urey experiment should still be taught because the basic results are still valid. The experiments show that organic molecules can form under abiotic conditions. Later experiments have used more accurate atmospheric compositions and achieved similar results. Even though origin-of-life research has moved beyond Miller and Urey, their experiments should be taught. We still teach Newton even though we have moved beyond his work in our knowledge of planetary mechanics. Regardless of whether any of our current theories about the origin of life turn out to be completely accurate, we currently have models for the processes and a research program that works at testing the models.
How textbooks could improve their presentations of the origin of life
Textbooks can always improve discussions of their topics with more up-to-date information. Textbooks that have not already done so should explicitly correct the estimate of atmospheric composition, and accompany the Miller-Urey experiment with a clarification of the fact that the corrected atmospheres yield similar results. Further, the wealth of new data on extraterrestrial and hydrothermal sources of biological material should be discussed. Finally, textbooks ideally should expand their discussions of other stages in the origin of life to include PNA and some of the newer research on self-replicating proteins. Wells, however, does not suggest that textbooks should correct the presentation of the origin of life. Rather, he wants textbooks to present this "icon" and then denigrate it, in order to reduce the confidence of students in the possibility that scientific research can ever establish a plausible explanation for the origin of life or anything else for that matter. If Wells's recommendations are followed, students will be taught that because one experiment is not completely accurate (albeit in hindsight), everything else is wrong as well. This is not good science or science teaching.
Table of Contents
- Icon 2 — Darwin's Tree of Life
- Icon 3 — Homology
- Icon 4 — Haeckel's Embryos
- Icon 5 — Archaeopteryx
- Icon 6 — Peppered Moths
- Icon 7 — Darwin's Finches
- Icons of Evolution? Conclusion
- Icons of Evolution? Figures
- Icons of Evolution? References
- "Icons" Critique — pdf versions
- Fatally Flawed Iconoclasm
- 10 Answers to Jonathan Wells's "10 Questions"
Miller-Urey Experiment – Definition & Detailed Explanation – Astrobiology Glossary
Table of Contents
What is the Miller-Urey Experiment?
The Miller-Urey Experiment is a groundbreaking scientific experiment that was conducted in 1953 by chemists Stanley Miller and Harold Urey. The experiment aimed to simulate the conditions of early Earth in order to investigate the origins of life. This experiment is considered one of the most important in the field of astrobiology and has had a significant impact on our understanding of the origins of life on Earth.
How was the Miller-Urey Experiment conducted?
In the Miller-Urey Experiment, Stanley Miller and Harold Urey created a closed system that mimicked the conditions of early Earth’s atmosphere. They used a mixture of gases such as methane, ammonia, hydrogen, and water vapor, which were believed to be present in the atmosphere of early Earth. The gases were circulated through a series of glass tubes and flasks, representing the oceans and atmosphere of the early Earth.
The gases were then subjected to electrical sparks to simulate lightning, which was thought to be a common occurrence in the early Earth’s atmosphere. After running the experiment for a week, Miller and Urey observed that the mixture of gases had produced a variety of organic compounds, including amino acids, which are the building blocks of proteins and essential for life.
What were the key findings of the Miller-Urey Experiment?
The key findings of the Miller-Urey Experiment were groundbreaking in the field of astrobiology. The experiment demonstrated that under the conditions of early Earth, simple organic molecules could spontaneously form from inorganic compounds. This provided evidence that the basic building blocks of life could have originated on Earth through natural processes.
The experiment also showed that the formation of complex organic molecules, such as amino acids, could occur in a relatively short period of time. This suggested that the origins of life may not have required millions of years, but could have happened relatively quickly under the right conditions.
What impact did the Miller-Urey Experiment have on the field of Astrobiology?
The Miller-Urey Experiment had a profound impact on the field of astrobiology. It provided experimental evidence to support the theory that life could have originated on Earth through natural processes. The experiment sparked further research into the origins of life and the conditions that may have existed on early Earth.
The findings of the Miller-Urey Experiment also inspired scientists to explore the possibility of life on other planets. By demonstrating that the basic building blocks of life could form under conditions similar to those found on early Earth, the experiment raised the possibility that life could exist elsewhere in the universe.
How has the Miller-Urey Experiment influenced our understanding of the origins of life on Earth?
The Miller-Urey Experiment has significantly influenced our understanding of the origins of life on Earth. The experiment provided evidence that the basic building blocks of life could have formed through natural processes on early Earth. This has led scientists to consider the possibility that life may be a common occurrence in the universe, given the right conditions.
The findings of the Miller-Urey Experiment have also led to further research into the origins of life and the conditions that may have existed on early Earth. Scientists continue to study the chemical reactions that could have led to the formation of complex organic molecules, with the goal of understanding how life first emerged on our planet.
What are some criticisms of the Miller-Urey Experiment?
While the Miller-Urey Experiment was groundbreaking in its findings, it has also faced criticism from some scientists. One criticism is that the experiment may not accurately reflect the conditions of early Earth’s atmosphere. Some researchers argue that the gases used in the experiment were not representative of the actual composition of the early Earth’s atmosphere.
Another criticism is that the experiment may have produced a higher concentration of organic compounds than would have been present on early Earth. Some scientists believe that the conditions of the experiment were too idealized and may not have accurately reflected the complexity of the early Earth environment.
Despite these criticisms, the Miller-Urey Experiment remains a landmark study in the field of astrobiology. It has paved the way for further research into the origins of life and has inspired scientists to explore the possibility of life beyond Earth. The experiment continues to be studied and referenced in scientific literature, as researchers seek to unravel the mysteries of how life first began on our planet.
SentinelMission.org
Hundreds of articles about space, astronauts, guides and free resources
Email: [email protected]
Follow Us !
Copyright © 2024 All Rights Reserved
Privacy policy
Cookie Policy

IMAGES
COMMENTS
Stanley Miller in 1999, posed with an apparatus like that used in the original experiment. At the time of the Miller–Urey experiment, Harold Urey was a Professor of Chemistry at the University of Chicago who had a well-renowned career, including receiving the Nobel Prize in Chemistry in 1934 for his isolation of deuterium [21] and leading ...
Study with Quizlet and memorize flashcards containing terms like What did the Miller-Urey experiment show? (Check all that apply.) -Life could be generated in the apparatus after one week -Essential biological molecules associated with living organisms could be generated in an "abiotic" environment. -Essential biological molecules associated with life cannot be generated in an "abiotic ...
Nov 28, 2024 · Miller-Urey experiment, experimental simulation conducted in 1953 that attempted to replicate the conditions of Earth’s early atmosphere and oceans to test whether organic molecules could be created abiogenically, that is, formed from chemical reactions occurring between inorganic molecules thought
Feb 2, 2023 · The Miller-Urey Experiment was a landmark experiment to investigate the chemical conditions that might have led to the origin of life on Earth. The scientist Stanley Miller, under the supervision of the Nobel laureate scientist Harold Urey conducted it in 1952 at the University of Chicago. They tried to recreate the conditions that could have […]
May 19, 2023 · The Miller-Urey experiment remains a significant milestone in our understanding of prebiotic chemistry and contributes to unraveling the complex puzzle of life’s origin. In conclusion, the Miller-Urey experiment’s hypothesis, steps, conclusions, and limitations provide valuable insights into the origin of life on Earth.
Miller and Urey examined the cooled water after a week and observed that 10-15% of the carbon was in the form of organic compounds. 2% of carbon had formed 13 amino acids. Yet, the Miller and Urey experiments were condemned by their fellow scientists. Also read: Origin Of Life. Criticism of the Miller Urey Experiment
This experiment provided the structure for later research into the origin of life. Despite many revisions and additions, the Oparin–Haldane scenario remains part of the model in use today. The Miller–Urey experiment is simply a part of the experimental program produced by this para-digm. WELLS BOILS OFF W ells says that the Miller–Urey exper-
Nov 22, 2006 · The Miller-Urey experiment is not relevant to the RNA world, because RNA was constructed from organic "building blocks" irrespective of how those compounds came into existence (Zubay and Mui, 2001). The evolution of RNA is a wholly different chapter in the story of the origin of life, one to which the validity of the Miller-Urey experiment is ...
Mar 22, 2024 · The Miller-Urey Experiment is a groundbreaking scientific experiment that was conducted in 1953 by chemists Stanley Miller and Harold Urey. The experiment aimed to simulate the conditions of early Earth in order to investigate the origins of life.
Miller–Urey experiment is not as conclusive: This experiment was a great achievement because it showed that that important prebiotic compound scan be abiotically synthesized in environments simulating “natural” conditions. But there are several findings which prove the conclusion of this experiment wrong! 1.