Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here

NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
NECO Chemistry Questions and Answers 2023. I will be showing you past Chemistry objectives and theory repeated questions for free in this post. You will also understand how NECO Chemistry questions are set and how to answer them.
The National Examinations Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and December/January respectively.
Table of Contents
NECO Chemistry Objectives and Essay Answers 2023 (Expo)
The 2023 NECO Chemistry expo will be posted here today 24th July during the NECO Chemistry examination. Keep checking and reloading this page for the answers.
NECO 2023 Chemistry Answers Loading.
OBJ Answers:
1-10: DEADADECAD
11-20: BAEDDBDBAE
21-30: CCDCABDDCD
31-40: EBEECEBCEE
41-50: BCCECDDADD
51-60: DABBDEAECA
————————————————————————————————————-
NECO Chemistry Questions and Answers For Practice
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
See: NECO Timetable
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
Recommended: How to check NECO Result
NECO Chemistry Questions And Answers 2023 (Paper 2)
Don’t worry about these NECO Chemistry Questions And Answers 2023. All you need to do is to keep on refreshing this page for the 2023 NECO Chemistry Questions And Answers for this year. It will be posted here in few minutes.
Tips on How to Pass 2023 NECO Chemistry Examinations
The following guidelines will help you pass the 2023 NECO Chemistry examination with flying colours.
Have a Target and Work Towards Actualizing it
You have decided to pass NECO Chemistry 2023 and I am sure of that. Now, the next thing you should do is set targets.
You have told yourself, “I will score A in NECO Chemistry 2023”, that’s not all. You need to plan on how to make it happen. Create a timetable and master plan to achieve your goals.
Get the Recommended Textbook on Chemistry for 2023 NECO Examination
Normally, NECO recommends books for the examination. But apart from NECO Literature in English where certain novels are compulsory, you are free to use any good Chemistry textbook to prepare for NECO 2023 exam.
Some textbooks are more difficult to understand. If you have any topic you are finding difficult to understand, then get a textbook that will simplify the topics and make life better for you.
Do not Skip Chemistry Examples and Exercise you Will Come Across While Reading:
Many candidates are fond of skipping exercises and even examples while studying textbooks. In fact, we like notebooks so much that we could ask, “can I read my notebook and pass NECO Chemistry 2023?” Don’t be scared of attempting exercises in Biology. Face the challenges.
If you have any questions about the NECO Chemistry Questions and Answers 2023 , kindly drop your question in the comment box.
Last Updated on July 25, 2023 by Admin
Related posts:

122 thoughts on “NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
Please when will the answer come up
I just need questions for the two
I am really greatfull for the coperation you showed to us most especially me if not for you guys thisneco would have been trouble so sincear thank you
WHERE IS THE ANSWER FOR THE OBJ QUESTIONS THAT IS UP THERE
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

Home » NECO » NECO Chemistry Questions & Answers 2023 (OBJ-Essay) Released
Home → NECO
Neco chemistry questions & answers 2023 (obj-essay) released.
See 2023 NECO Chemistry Answers & Questions Here.
The Neco chemistry answers for 2023 questions can now be seen here. The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm.
This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write.
Here, we will be posting the neco chemistry questions for candidates that will participate in the examination. Note that below is the questions from past questions and answers that we feel are likely questions for SSCE preparation.

NECO Chemistry Answers 2023.
1. a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca.
2. a) (i) Define nuclear fission. (ii) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1. Explain briefly the effect of each of the following changes on the equilibrium composition:
- increase in the concentration of B;
- decrease in pressure of the system;
- addition of catalyst.
b) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
c) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2.
3. a) (i) Define saturated solution. (ii) Distinguish between dative bond and covalent bond. (iii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in: hydrogen fluoride; argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give reason for your answer.
(b) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p. [ standard vapour pressure of water at 18°C = 15 mmHg]
4. a) (i) Define ionic bond. (ii) What type of bond(s) exist(s) in: magnesium oxide; ammonium ion?
b) (i) Determine the oxidation number of sulphur in Na2S2O3. (ii) State Faraday’s first law. (iii) Give one example each of: acid salt; base salt.
c) (i) Name the type of energy change that occurs in each of the following processes; I2(s) ———> I2(g); C1(g) + e- ——> C1-(g). (ii) State the effect of each of the following aqueous solutions on litmus paper: Na2SO4(aq); AlC13(aq) (iii) Define the term efflorescence. (iv) Give two uses of activated charcoal.
d) (i) State one use of each of the following processes in the chemical industry: hydrogenation of vegetable oil; cracking; esterification. (ii) Calculate the amount of silver deposited in moles when 10920 coulombs of electricity is passed through a solution of a silver salt. [Faraday constant = 96500 C mol-1]
5. a) (i) Define in terms of electron transfer I. oxidizing agent; II. reducing agent. (ii) Write a balanced equation to show that carbon is a reducing agent. (iii) State the change in oxidation number of the specie that reacted with carbon in 5 (a)(ii).
b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H2S to form sulphur. Using the information provided: identify gas X; state two properties exhibited by X; give two uses of X.
c) Consider the following substances: (1) sodium; (2) lead (II) iodide; (3) hydrogen; (4) magnesium; (5) oxygen. Which of the substances (i) conducts electricity? (ii) is produced at the cathode during electrolysis of H2SO4(aq)? (iii) corresponds to the molecular formula AB2 ? (iv) is an alkaline earth metal?
d) (i) Define the term salt. (ii) Mention two types of salt. (iii) Give an example of each of the salts mentioned in 5(d)(ii) above.
e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution. (i) Write a balanced equation for the reaction. (ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
Note: There is nothing like Neco chemistry Expo online. Neco Ssce candidates are to desist from patronizing online fraudsters / vendors who says they can provide such services as they are not real.
Neco Chemistry Objective Questions 2023.
NOTE: There is nothing like Neco chemistry expo online. Do not be deceived by fraudsters posing fake NECO chemistry answers on the internet.
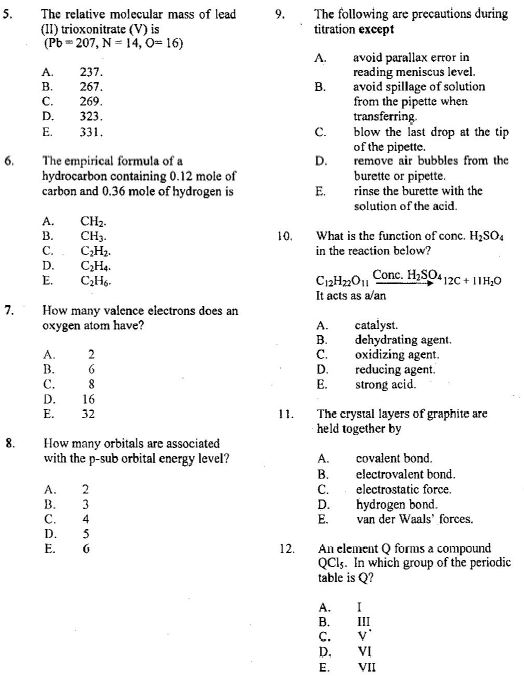
13. At a particular temperature and pressure, 15.0 g of CO2 occupy 7.16 liters. What is the volume of 12.0 g of CH4 at the same temperature and pressure? A.
Keep following this page. If you have any questions, endeavour to use the comment box below…
Industrial Action: ASUU, FG Schedule Another Meeting for November 20
Neco animal husbandry answers 2023 [essay-obj-practical] is out, other recommended posts for you, neco bece 2023 registration form & time table is now out, neco data processing answers 2023 practicals is out, comments (33).
Are the questions legitimate
It was very useful Please add me up
Can i get 2022 neco chemistry theory…
This is very good
You are in point but not at all will people read there is expo
Can I get chemistry practical question of 2023?
We should all read our books & not rely on expo
Why u con dey this site
Today chemistry answers
Yes I agree wholeheartedly agree with you.
So what are u doing here U are cross checking ur work nonsense
I hope the questions are not fake
I need to join this group so that I can excel in my exam. So lovely. Thanks.
I want to join this group
I really need to join this group
I need the Neco 2021 questions and answers
How about the answers
Obj and theory answers chemistry pls
Objective pls
How can I join please
I loved to join this group,this website is great, thanks so much
These chemistry questions above, the correct ones for neco 2021?
I wish to joined dis group
For chemistry obj n essay
please i need neco 2022 answers
I just love unn-edu.info. I wrote Agric exams neco today main paper and can you imagine that some of the questions they posted here came out today . So I advise anything that is posted here should be given utmost attention.
Oyebode precious read your books, stop looking for expo
Where are the solution for those questions above.
The answer for this
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

JOIN OUR WAEC & NECO WHATSAPP SAPP GROUP

Chemistry-Obj USE THIS OBJ FOR A1.. FEW CORRECTION MADE 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCADDECD 31-40: EBEEBEBCEE 41-50: BCCECEDADD 51-60: DABBDEAECA
CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent (1aiii) Soaps are made from natural products while detergents are made from synthetic products. (1aiv) Detergents is for household cleaning and laundry purposes (1bi) Number of neutrons = Mass number (A) – Atomic number (Z) I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number) Number of neutrons = 23 – 11 = 12 II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number) Number of neutrons = 39 – 19 = 20 (1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol Now, let’s calculate the mass of oxygen needed: First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium. (1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l) From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂). Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol Now, let’s calculate the number of moles of CaCO₃ in 50g: Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol. Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium. (1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation. (1ciii) (I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃. The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol. (II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O. The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O) Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol) Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C Substituting the values to calculate the mass of silver deposited: Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)

(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol (3aii) (i) Fermentation (ii) Ethylene hydration (3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72 (3bi) 1s², 2s², 2p⁴ (3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless (3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically (3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O (3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system. (3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g) (3ciii) Redox reaction. (3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. (4aii) 15/345 = Solubility *25/1000 Solubility =1000*15/25*345=15000/8625 Solubility = 1.79mol/dm³ (4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆ (4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures (4bii) (i) Noble gases (ii) Carbon (iv) oxide (4biii) H₂SO₄ —-> 2H+ + SO₄²⁻ 1 mole of H₂SO₄ = 2 mole of H⁺ 0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions (4biv) (i) Dative bonding (ii) Hydrogen bonding (4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures. (ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Completed!!

I’m a blogger living in Nigeria. I like to share education guides and information from various sources. I created Jambclass to serve as a platform to disseminate quality, credible and dependable information regarding various ways to excel academically. I strive to keep Nigerian youths and students informed. I started this Blog with the Vision To Inspire and Empower Young Persons; helping them Realize and Maximize their Potential.

Leave a Reply
Name (required)
Email (required, but never shared)
NOTE:- Your comment will appear after it has been approved by an admin .
Home | Recent Posts | Pages
About Jambclass
Copyright © 2022 Jambclass
My Scholarship Baze
2023 neco chemistry questions and answers, 2023 neco chemistry essay answers.
Here is the neco Chemistry questions and answers

(1ai) (CHOOSE ANY BEST 3) (i) Sulphur is used to produce sulphur(IV)oxide for manufacturing tetraoxosulphate(VI)acid (ii) Sulphur is used in the vulcanization of rubber. (iii) Sulphur and some of its products are used as fungicides and insecticides for spraying crops. (iv) Sulphur is used to manufacture the bleaching agent used in the pulp and paper industry.
(1aii) (CHOOSE ANY BEST 2) (i) Hydrogen Sulphide is a colorless gas with a repulsive smell like that of a rotten egg. (ii) It is very poisonous. (iii) It is about 1.18 times denser than air. (iv) It burns with a pale blue flame.
(1aiii) Coming…..
???? 2023 NECO CHEMISTRY ESSAY ANSWERS ????
(1aiii) Coming….. (3) (i) Classifying the alkanols: – Butan-2-ol: secondary alkanol – 2-methylpropanol: secondary alkanol – 2-methylpropan-2-ol: tertiary alkanol
(ii) Two methods to prepare ethanol commercially: – Fermentation: Ethanol can be produced by the fermentation of sugars using yeast. This is commonly used to produce alcoholic beverages and biofuels. – Hydration of ethene: Ethanol can be produced by the hydration of ethene (ethylene) in the presence of a catalyst, such as phosphoric acid.
(iii) To calculate the relative molecular mass of Z, we need to compare the rates of effusion or diffusion of Z and hydrogen. The rate of effusion/diffusion is inversely proportional to the square root of the molar mass. Since hydrogen diffuses 6 times as fast, the molar mass of Z is 6 times larger than the molar mass of hydrogen. Therefore, the relative molecular mass of Z is 6 * 2 = 12.
(bi) The electronic configuration of oxygen using s, p, d, f notation is 1s2 2s2 2p4.
(ii) Three physical properties of oxygen: (i) Oxygen is a colorless and odorless gas. (ii) It is slightly soluble in water. (iii) Oxygen supports combustion and is necessary for respiration.
(iii) Two rules for naming alkenes: 1. Alkenes must have the ending “-ene” in their name. 2. The longest continuous carbon chain containing the double bond is used as the base name, and the position of the double bond is indicated by the lowest possible number.
(iv) Equation for the oxidation reaction of ethene: Ethene + Oxygen → Carbon Dioxide + Water C2H4 + O2 → CO2 + H2O
(ci) Endothermic reaction: A reaction that absorbs heat (energy) from the surroundings, resulting in a decrease in temperature.
(ii) Equation for the laboratory preparation of hydrogen using dilute tetraoxosulphate(VI) acid and Zinc: Zn + H2SO4 → ZnSO4 + H2
(iii) The type of reaction involved in (cii) is a displacement reaction, where zinc displaces hydrogen from the acid to form hydrogen gas.
(iv) Three uses of hydrogen: (i) Hydrogen is used as a fuel for combustion engines and fuel cells. (ii) It is used in the production of ammonia for fertilizer and other chemicals. (iii) Hydrogen is used in the hydrogenation of oils and fats in the food industry. (1ai) (CHOOSE ANY BEST 3) (i) Sulphur is used to produce sulphur(IV)oxide for manufacturing tetraoxosulphate(VI)acid (ii) Sulphur is used in the vulcanization of rubber. (iii) Sulphur and some of its products are used as fungicides and insecticides for spraying crops. (iv) Sulphur is used to manufacture the bleaching agent used in the pulp and paper industry.
(1aiii) Soaps are made from natural ingredients, typically fats or oils, and an alkali (such as sodium hydroxide or potassium hydroxide) in a process called saponification. WIHILE Detergents are synthetic (man-made) cleaning agents composed of various chemicals, including surfactants.
Soaps are effective at removing dirt, grease, and oil, but they may not perform as well in hard water conditions. WHILE Detergents are generally more effective than soaps in all water conditions, including hard water, due to their ability to work with the mineral ions present.
(1aiv) (CHOOSE ANY BEST 1) (i) Detergents are used in various personal care products, such as shampoos, body washes, and hand soaps. (ii) Detergents are specifically designed for cleaning dishes, cutlery, glasses, and cookware. (iii) Detergents are used for general household cleaning tasks.
Related posts:
- Jamb Chemistry past questions and answers PDF downoad
NECO CHEMISTRY PRACTICAL ANSWERS 2021
- June 23rd JAMB CHEMISTRY QUESTIONS AND ANSWERS 2021
- NECO 2021 CHEMISTRY VERIFIED ANSWERS
- WAEC CHEMISTRY ANSWERS 2020 CONFIRMED
- Neco Chemistry questions and answers 2023
- NECO CHEMISTRY ANSWERS 2020
- waec Chemistry Questions and answers 2023
- Neco Chemistry practical answers 2022
- JAMB CHEMISTRY 2023 LIKELY QUESTION
Related Posts

NECO FISHERY PRACTICAL QUESTIONS 2021

Mathematics Neco Questions 2024


About The Author
MyScholarshipBaze
I love surfing the web and providing great information for my readers. I am an Editor At Myscholarshipbaze.com
Leave a Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
Privacy Overview

Fast & Easy Contact

Chemistry Questions and Answers for NECO 2023
EXAM : Neco 2023
SUBJECT : chemistry
DATE: 24 th July 23

OBJ CHEMISTRY 1-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCABDDCD 31-40: EBEECEBCEE 41-50: BCCECDDADD 51-60: DABBDEAECA Completed. ======================= QUESTION CHEMISTRY (1) (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent (1aiii) Soaps are made from natural products while detergents are made from synthetic products. (1aiv) Detergents is for household cleaning and laundry purposes (1bi) Number of neutrons = Mass number (A) - Atomic number (Z) I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number) Number of neutrons = 23 - 11 = 12 II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number) Number of neutrons = 39 - 19 = 20 (1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol Now, let's calculate the mass of oxygen needed: First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium. (1biii) CaCO₃(s) + 2 HCl(aq) ---> CaCl₂(aq) + CO₂(g) + H₂O(l) From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂). Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol Now, let's calculate the number of moles of CaCO₃ in 50g: Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol. Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride. (1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium. (1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound's origin or method of preparation. (1ciii) (I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃. The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol. (II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O. The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O) Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol) Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol. ========================================= QUESTION CHEMISTRY (2) (2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday) Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C Substituting the values to calculate the mass of silver deposited: Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C Mass of silver deposited ≈ 27.8g (2aii) (i) Electrode surface area (ii) Electrolyte temperature (2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq) (2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ ----> Mn²⁺ + 4H₂O(l) (2bi) (2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion. (2biii) Faraday's second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte. (2biv) (i) Charcoal (ii) Coal (2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum) ===================================================== QUESTION CHEMISTRY (3) (3ai) (i) Butan-2-ol - Secondary alkanol (ii) 2-methylpropanol - Primary alkanol (iii) 2-methylpropan-2-ol - Tertiary alkanol (3aii) (i) Fermentation (ii) Ethylene hydration (3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2x36 M = 72 (3bi) 1s², 2s², 2p⁴ (3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless (3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically (3biv) C₂H₄ + O₂ ---> 2CO₂ + 2H₂O (3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system. (3cii) Zn(s) + H₂SO₄(aq) ---> ZnSO₄(aq) + H₂(g) (3ciii) Redox reaction. (3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer ===================================================== QUESTION CHEMISTRY (4) (4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. (4aii) 15/345 = Solubility *25/1000 Solubility =1000*15/25*345=15000/8625 Solubility = 1.79mol/dm³ (4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆ (4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures (4bii) (i) Noble gases (ii) Carbon (iv) oxide (4biii) H₂SO₄ ----> 2H+ + SO₄²⁻ 1 mole of H₂SO₄ = 2 mole of H⁺ 0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro's constant No. of H⁺ = Mole * Avogadro's constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions (4biv) (i) Dative bonding (ii) Hydrogen bonding (4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures. (ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery. ===================================================== QUESTION CHEMISTRY (5) (5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion (5aii) (i) K₂O (ii) MgO (5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals (5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has (5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65 (5bi) (i) Temperature (ii) concentration (iii) surface area (5bii) The law states that energy can neither be created nor destroyed in and isolated system. (5biii) (i) burning of wood (ii) neutralization reaction 5C ===================================================== QUESTION CHEMISTRY (6)
Join our fb group to see free answer, post a comment, waec quick links.
💬 WAEC Registration
JAMB QUICK LINKS
💬 JAMB CAPS
Popular Posts

Neco GCE 2024 general mathematics questions and answers
Random posts.

Neco Gce 2024 igbo hausa and yoruba questions and answers

Neco 2024 CRS questions and answers
Footer menu widget.
- PRIVACY POLICY
Emmalex Stories
The Home of True Life Stories, Cultural Stories, Educational Stories, Adventure, Jokes, and Lots More
2023 NECO Chemistry Questions and Answers (Essay & Obj)
By Engr. Emmalex
2023 neco chemistry questions and answers, ( essay and objectives ).
2023 NECO Chemistry Answers: Get NECO 2023 Chemistry Questions and answers for 2023 Paper 2 and 3 ( Essay and Objectives ) .
We have the 2023 NECO Chemistry Questions and Answers Expo, so count yourself lucky to be here on our website, where we provide real and verified answers.
We are best EXAM runs website, so subscribe with us today for your NECO Examination Questions and Answers Now.
Note: Our Exam Answers come 3hours before the exam time (2023 NECO Questions and Answers Expo and Runs ) . Click here to Subscribe Now!
Neco 2023 chemistry objectives solutions, neco 2023 chemistry essay solutions.
Keep Refreshing this page because More answers will be posted soon .
NECO 2023 Chemistry Practical Solutions
NECO CHEMISTRY-PRACTICALS ANSWERS for 2023
(1a) [TABULATE]
Burette Reading (cm³) | Rough | Titration I | Titration II Final Reading (cm³) | 21.50 | 43.00 | 22.00 Initial Reading (cm³) | 0.00 | 21.50 | 00.00 Volume of A used (cm³) | 21.50 | 21.50 | 22.00
(1ai) Average Volume of acid used = 21.50 + 21.50/2 = 21.50cm³
(1aii) Solution B[Sodium trioxocarbonate (iv)] feels soapy
(1bi) Conc. B in mol/dm³ =? Ca= 0.04, Va= 21.50cm³, na= 1 Cb= ? , Vb= 25.00cm³ nb= 1
CaVa/CbVb = na/nb = 0.04×21.50/Cb×25.00 = 1/1 Cb= 0.04×21.50/25.00 = 0.86/25.00 Cb= 0.0344mol/dm³
(1bii) Conc. of B in g/dm³ = Conc. of B in mol/dm³ × molar mass of B
Molar mass of B(NaCO₃)= (23×2) + 12 + (16×3) = 106
:. Conc. of B in g/dm³ = 0.0344×106 = 3.6g/dm³
(1biii) 1 mole of Na₂Co₃ = 1mole of Na₂So₄
:. 0.0344 mole of Na₂Co₃ = 0.0344 mole of Na₂So₄ № of mole= mass/ molar mass
Molar mass of Na₂So₄= (23×2)+32+(16×4)=142 :. 0.0344 = mass/142 Mass=142×0.0344=4.9g
(1biv) 1 mole of Na₂Co₃= 1 mole of CO₂
0.0344 mole of Na₂Co₃ = 0.0344 mole of CO₂ :. № of mole = volume/ molar volume = 0.0344 = volume of CO₂/22400cm³
(2ai) [TABULATE]
=TEST= C + heat
=OBSERVATION= Formation of yellow flame
=INFERENCE= Na⁺ present
(2aii) =TEST= C + distilled water + shake
=OBSERVATION= It readily dissolve in water
=INFERENCE= C is a soluble in water
(2aiii) =TEST= Solution C + litmus paper
=OBSERVATION= It changes moist red litmus to blue and no effect blue
=INFERENCE= Solution C is alkaline
(2bi) =TEST= 1st portion of solution C + barium chloride
=OBSERVATION= White precipitate formed
=INFERENCE= CO₃²⁻ , SO₄²⁻ , S²⁻ or SO₃²⁻
(2bii) =TEST= Add dilute HCl to product in (bi)
=OBSERVATION= White precipitate dissolved
=INFERENCE= CO₃ , SO₃²⁻ or S₃²⁻
(2biii) =TEST= To the second portion add phenolphthalein
=OBSERVATION= Purple colour observed
=INFERENCE=
C is alkaline
(3ai) Sulphur (iv) oxide and hydrogen sulphide.
(3aii) Liberation of hydrogen gas with pop sound and formation of green iron (ii) chloride solution
(3aiii) Spatula, beaker, weighing balance, standard volumetric flask
(3bi) Solution turns red
(3bii) Turns from brown to green
A colourless, odourless, acidic gas liberated which turns blue litmus paper red and turn lime water milky.
If you want to get the 2023 WAEC , NECO or NABTEB Questions and Answers 3Hours before the Exam Date and time, Click here . and Subscribe to our NECO Exam answers.
NECO 2023 Chemistry Practical Questions
TO SUBSCRIBE FOR 2023 WAEC, NECO & NABTEB EXAM ANSWERS VIA WHATSAPP JOIN OUR GROUP
LIVE NECO QUESTIONS AND ANSWERS SUBSCRIBE NOW
We are the Top Examination Expo Website for WAEC, NECO and NABTEB Exams Runs and Verified Exam Answers provider in Nigeria, Ghana, Gambia, Sierra Leone , Liberia, Cameron, etc.
We have Questions and Answers in all Subjects for WAEC, NECO and NABTEB. Join Our Whatsapp Group Now to gain access, Click Here To Join.
Do you know that if you are seeking admission into University, Polytechnic or College Of Education, you need to have A or B in your WAEC, NECO or NABTEB Result? Because it gives you a better chance than those WITH C.
If you really want to have A or B in your results, make sure you subscribe to our exam runs, that is the only way to secure your admission.
Note: Our WAEC/NECO/NABTEB Exam Expo and RUNZ Answers come 3hours before the exam time (Verified Answers).
To Get Our 2023 WAEC, NECO or NABTEB Exams Questions and Answers Click Here To Get
Click Here for other Subjects
Related post, 2023 neco chemistry questions and answers (essay & obj), 2023 neco economics questions and answers (essay & obj), 2023 neco english language answers for essay & objective, leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Recent Posts You Missed
Unoka in things fall apart by chinua achebe, zero account – rekindle your fire, jesus really does love you, how i met the love of my life.
EXAMCLASS.NET BEST WEBSITE FOR JAMB RUNZ,WAEC RUNZ, NECO RUNZ,SCHOOL NEWS, UPDATES
Your #no 1 best website for waec runz | jamb cbt runz | neco runz | gce runz and school news, for easy & fast contact +2347046585189 || call 07046585189, 2023 neco gce chemistry (essay & obj) answers.
December 6, 2023 Mr Class Neco Gce 0

NECO GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS 2023
Neco GCE 2023 Chemistry Expo > All NECO GCE candidates are hereby informed that our platform has given go ahead for 2022 Neco GCE per subject subscription for the 2023/2024 ongoing Neco SSCE November/December External exam.
This is to say that Neco Gce Chemistry Expo 2023 Runs Questions and Answers Subscription has commenced online so you can pay. Be part of your success by following the guidelines provided on this article.
NECO GCE 2023/2024 chemistry theory EXPO SUBSCRIPTION
Our subscription price list for whatsapp message..
(i) Per Subject: N600 each (ii) (iii) Maths & English: N1200 each
Our Subscription Price List For Password Answers.
(i) Per Subject: N500 each (ii) practicals Per Subject: N500 each (iii) Maths & English: N1000 each
CLICK HERE FOR ANSWERPAGE
Send (i) Payment name/Your name. (ii) Subject Name (e.g Chemistry) (iii) MTN Recharge Card Pin(s) (iv) Phone number to 09068045697
With MR classic man , Your Success In Sure.
Remember Success is not a must, is an Option. Don’t let this little amount of money make you fail this exam. NECO GCE 2023 EXPO On today Chemistry ANSWERS
WARNING :-Please Don’t Even Come for Free Answers, because it Would’nt be Posted. Take me Serious This Time.
Make Sure you Subscribe if you Don’t Want to be on Hot Seat
Neco Gce Chemistry Expo 2023 – Neco Gce Chemistry Runs 2023 / Neco Gce Chemistry Objective and Essay Answers / Neco Gce Chemistry Questions and Answers Runz 2023/2024.
If you discovered this amusing webpage, you must be very lucky enough, because a lot of people have been seeking to know us, but it’s not easy as you think. Regardless of wherever you got our attention from, you are welcome to the best Nigeria exam solution provider portal.
This article is written to expose our mode of operation regarding the ongoing Neco SSCE November/December External exam. Mr classic mission is for you to make excellent results in the Neco SSCE exam. I have not seen where our Candidates after writing various exams we help, reportedly fails, its never our motto.
Get Neco Gce Chemistry Objective and Essay Answers sent to you via Whatsapp or direct text message few hours before the commencement of any exam as stated in the timetable.
NB : We have Password link Answers delivery, but The best we provide our selfs is via Whatsapp platform. 2023 NECO GCE Chemistry (Essay & OBJ) Answers
On Wednesday December 6th 2023, chemistry papers will be written as confirmed by the Timetable released by Neco exam board. Are you one of the candidates seeking external help for the above mentioned subjects, don’t hesitate to reckon on Examclass.net for the assistance. We have not failed and can never fail.
Thinking of how to Get Neco Gce Chemistry Expo 2023 Runs Questions and Answers, Mr classic website is the best to give you all the necessary, unique and verified answers to them.
In order to be part of the candidates who wish to subscribe, Neco Gce Chemistry Objective and Essay will cost the sum 600 only. It is left for the candidate to choose method of payment. NECO GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS 2023
How to Get NECO Gce Chemistry Answers Contact Mr classic on 090 6804 5697.
Send your Mtn Recharge Card Pin to our line 09068045697 on WhatsApp or do mobile transfer or pos vtu by requesting for Mr classic mtn number on Same WhatsApp.

Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2024 | WordPress Theme by MH Themes
Content protected!!

IMAGES
COMMENTS
NECO Chemistry Questions And Answers 2023 (Paper 2) Don't worry about these NECO Chemistry Questions And Answers 2023. All you need to do is to keep on refreshing this page for the 2023 NECO Chemistry Questions And Answers for this year. It will be posted here in few minutes. Tips on How to Pass 2023 NECO Chemistry Examinations
See 2023 NECO Chemistry Answers & Questions Here. The Neco chemistry answers for 2023 questions can now be seen here. The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm. This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will ...
Neco 2023 Chemistry Objective And Theory Questions And Answers By Jambclass on July 24th, 2023. Neco. 0 . Chemistry-Obj USE THIS OBJ FOR A1.. FEW CORRECTION MADE 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCADDECD 31-40: EBEEBEBCEE 41-50: BCCECEDADD 51-60: DABBDEAECA. CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization ...
2023 NECO CHEMISTRY ESSAY ANSWERS Here is the neco Chemistry questions and answers (1ai) (CHOOSE ANY BEST 3) (i) Sulphur is used to produce sulphur(IV)oxide for manufacturing tetraoxosulphate(VI)acid (ii) Sulphur is used in the vulcanization of rubber. (iii) Sulphur and some of its products are used as fungicides and insecticides for spraying crops. (iv) Sulphur is used to manufacture the ...
The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass.
The National Examination Council of Nigeria (NECO) Examination is around the corner. This article provides the readers with the information on how to download NECO Chemistry Past Questions and Answers. NECO Chemistry Questions are essential for you to know if you want to pass the NECO SSCE Chemistry Exams 2023.
2023 NECO Chemistry Answers: Get NECO 2023 Chemistry Questions and answers for 2023 Paper 2 and 3 (Essay and Objectives). We have the 2023 NECO Chemistry Questions and Answers Expo, so count yourself lucky to be here on our website, where we provide real and verified answers.
[pyear] Chemistry science answers. How to Use NECO Chemistry Questions June/July Answers. NECO Chemistry June/July theory and objective answers i is getting ready for you. We assure you that the solution is the best you can ever fine. All the steps and working are properly shown to be according to the marking scheme. Tips for Excelling in NECO ...
Get Free Live 2023 NECO GCE Chemistry (CHEM) OBJ & THEORY Questions and Answers Free of Charge | NECO Nov/Dec Free CHEMISTRY (Objectives and Theory) Questions and Answers EXPO Room (6th December, 2023).
Neco Gce Chemistry Expo 2023 - Neco Gce Chemistry Runs 2023 / Neco Gce Chemistry Objective and Essay Answers / Neco Gce Chemistry Questions and Answers Runz 2023/2024. If you discovered this amusing webpage, you must be very lucky enough, because a lot of people have been seeking to know us, but it's not easy as you think.