Systematic Reviews
Aims and scope.
The journal publishes high quality systematic review products including systematic review protocols, systematic reviews related to a very broad definition of human health, (animal studies relevant for human health), rapid reviews, updates of already completed systematic reviews, and methods research related to the science of systematic reviews, such as decision modelling. At this time Systematic Reviews does not accept reviews of in vitro studies. The journal also aims to ensure that the results of all well-conducted systematic reviews are published, regardless of their outcome. (You can now also prospectively register these reviews on PROSPERO.)


Trending articles
Click here to view which articles have been shared the most in the last month!
- Most accessed
Associations between HEXACO personality traits, substance use disorders, and behavioral addictions: a protocol for a comprehensive systematic review and meta-analysis
Authors: Farangis Sharifibastan, Eilin Kristine Erevik, Katharina Teresa Enehaug Morken and Ståle Pallesen
An updated network meta-analysis of non-pharmacological interventions for primary hypertension in adults: insights from recent studies
Authors: Ziwen Chen, Qifu Li, Tao Xu, Xueli Zhou, Yunjie Shu, Taipin Guo and Fanrong Liang
Diagnostic accuracy of metagenomic next-generation sequencing in pulmonary tuberculosis: a systematic review and meta-analysis
Authors: Yajie You, Ying meng Ni and Guochao Shi
Hybrid Service Delivery for voluntary, community and social enterprise organisations working with adults with learning disabilities and/or autism: a realist review protocol
Authors: Danielle Varley, Kris Southby, Joanne Trigwell, Sally SJ Brown, Nicola Lines, Amy Hearn and Anne-Marie Bagnall
Tranexamic acid in hip and spine surgery for children with cerebral palsy — a PRISMA-compliant scoping review
Authors: Daniel Gould, Haoze Cui, Norine Ma, George Chalkiadis, Andrew Davidson, Kerr Graham and Erich Rutz
Most recent articles RSS
View all articles
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement
Authors: David Moher, Larissa Shamseer, Mike Clarke, Davina Ghersi, Alessandro Liberati, Mark Petticrew, Paul Shekelle and Lesley A Stewart
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
Authors: Matthew J. Page, Joanne E. McKenzie, Patrick M. Bossuyt, Isabelle Boutron, Tammy C. Hoffmann, Cynthia D. Mulrow, Larissa Shamseer, Jennifer M. Tetzlaff, Elie A. Akl, Sue E. Brennan, Roger Chou, Julie Glanville, Jeremy M. Grimshaw, Asbjørn Hróbjartsson, Manoj M. Lalu, Tianjing Li…
The Editorial to this article has been published in Systematic Reviews 2021 10 :117
PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews
Authors: Melissa L. Rethlefsen, Shona Kirtley, Siw Waffenschmidt, Ana Patricia Ayala, David Moher, Matthew J. Page and Jonathan B. Koffel
Drug discontinuation before contrast procedures and the effect on acute kidney injury and other clinical outcomes: a systematic review protocol
Authors: Swapnil Hiremath, Jeanne Françoise Kayibanda, Benjamin J. W. Chow, Dean Fergusson, Greg A. Knoll, Wael Shabana, Brianna Lahey, Olivia McBride, Alexandra Davis and Ayub Akbari
Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study
Authors: Wichor M. Bramer, Melissa L. Rethlefsen, Jos Kleijnen and Oscar H. Franco
Most accessed articles RSS
Sign up to receive article alerts
Systematic Reviews is published continuously online-only. We encourage you to sign up to receive free email alerts to keep up to date with all of the latest articles by registering here .
Peer review mentoring
The Editors endorse peer review mentoring of early career researchers. Find out more here
Article collections
Thematic series The role of systematic reviews in evidence-based research Edited by Professor Dawid Pieper and Professor Hans Lund
Thematic series Canadian Task Force on Preventive Health Care Evidence Reviews Edited by Assoc Prof Craig Lockwood
Thematic series Automation in the systematic review process Edited by Prof Joseph Lau
Thematic series Five years of Systematic Reviews
View all article collections
Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
Citation Impact 2023 Journal Impact Factor: 6.3 5-year Journal Impact Factor: 4.5 Source Normalized Impact per Paper (SNIP): 1.919 SCImago Journal Rank (SJR): 1.620
Speed 2023 Submission to first editorial decision (median days): 92 Submission to acceptance (median days): 296
Usage 2023 Downloads: 3,531,065 Altmetric mentions: 3,533
- More about our metrics
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT)
The World's #1 Systematic Review Tool

See your systematic reviews like never before
Faster reviews.
An average 35% reduction in time spent per review, saving an average of 71 hours per review.
Expert, online support
Easy to learn and use, with 24/7 support from product experts who are also seasoned reviewers!
Seamless collaboration
Enable the whole review team to collaborate from anywhere.
Suits all levels of experience and sectors
Suitable for reviewers in a variety of sectors including health, education, social science and many others.
Supporting the world's largest systematic review community
See how it works.
Step inside Covidence to see a more intuitive, streamlined way to manage systematic reviews.
Unlimited use for every organization
With no restrictions on reviews and users, Covidence gets out of the way so you can bring the best evidence to the world, more quickly.
Covidence is used by world-leading evidence organizations
Whether you’re an academic institution, a hospital or society, Covidence is working for organizations like yours right now.
See a list of organizations already using Covidence →

How Covidence has enabled living guidelines for Australians impacted by stroke
Clinical guidelines took 7 years to update prior to moving to a living evidence approach. Learn how Covidence streamlined workflows and created real time savings for the guidelines team.

University of Ottawa Drives Systematic Review Excellence Across Many Academic Disciplines
University of Ottawa

Top Ranked U.S. Teaching Hospital Delivers Effective Systematic Review Management
Top Ranked U.S. Teaching Hospital
See more Case Studies

Better systematic review management
Head office, working for an institution or organisation.
Find out why over 350 of the world’s leading institutions are seeing a surge in publications since using Covidence!
Request a consultation with one of our team members and start empowering your researchers:
By using our site you consent to our use of cookies to measure and improve our site’s performance. Please see our Privacy Policy for more information.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

Systematic Reviews
Describes what is involved with conducting a systematic review of the literature for evidence-based public health and how the librarian is a partner in the process.
Several CDC librarians have special training in conducting literature searches for systematic reviews. Literature searches for systematic reviews can take a few weeks to several months from planning to delivery.
Fill out a search request form here or contact the Stephen B. Thacker CDC Library by email [email protected] or telephone 404-639-1717.
Campbell Collaboration
Cochrane Collaboration
Eppi Centre
Joanna Briggs Institute
McMaster University
PRISMA Statement
Systematic Reviews – CRD’s Guide
Systematic Reviews of Health Promotion and Public Health Interventions
The Guide to Community Preventive Services
Look for systematic reviews that have already been published.
- To ensure that the work has not already been done.
- To provides examples of search strategies for your topic
Look in PROSPERO for registered systematic reviews.
Search Cochrane and CRD-York for systematic reviews.
Search filter for finding systematic reviews in PubMed
Other search filters to locate systematic reviews
A systematic review attempts to collect and analyze all evidence that answers a specific question. The question must be clearly defined and have inclusion and exclusion criteria. A broad and thorough search of the literature is performed and a critical analysis of the search results is reported and ultimately provides a current evidence-based answer to the specific question.
Time: According to Cochrane , it takes 18 months on average to complete a Systematic Review.
The average systematic review from beginning to end requires 18 months of work. “…to find out about a healthcare intervention it is worth searching research literature thoroughly to see if the answer is already known. This may require considerable work over many months…” ( Cochrane Collaboration )
Review Team: Team Members at minimum…
- Content expert
- 2 reviewers
- 1 tie breaker
- 1 statistician (meta-analysis)
- 1 economist if conducting an economic analysis
- *1 librarian (expert searcher) trained in systematic reviews
“Expert searchers are an important part of the systematic review team, crucial throughout the review process-from the development of the proposal and research question to publication.” ( McGowan & Sampson, 2005 )
*Ask your librarian to write a methods section regarding the search methods and to give them co-authorship. You may also want to consider providing a copy of one or all of the search strategies used in an appendix.
The Question to Be Answered: A clearly defined and specific question or questions with inclusion and exclusion criteria.
Written Protocol: Outline the study method, rationale, key questions, inclusion and exclusion criteria, literature searches, data abstraction and data management, analysis of quality of the individual studies, synthesis of data, and grading of the evidience for each key question.
Literature Searches: Search for any systematic reviews that may already answer the key question(s). Next, choose appropriate databases and conduct very broad, comprehensive searches. Search strategies must be documented so that they can be duplicated. The librarian is integral to this step of the process. Before your librarian creates a search strategy and starts searching in earnest you should write a detailed PICO question , determine the inclusion and exclusion criteria for your study, run a preliminary search, and have 2-4 articles that already fit the criteria for your review.
What is searched depends on the topic of the review but should include…
- At least 3 standard medical databases like PubMed/Medline, CINAHL, Embase, etc..
- At least 2 grey literature resources like Clinicaltrials.gov, COS Conference Papers Index, Grey Literature Report, etc…
Citation Management: EndNote is a bibliographic management tools that assist researchers in managing citations. The Stephen B. Thacker CDC Library oversees the site license for EndNote.
To request installation: The library provides EndNote to CDC staff under a site-wide license. Please use the ITSO Software Request Tool (SRT) and submit a request for the latest version (or upgraded version) of EndNote. Please be sure to include the computer name for the workstation where you would like to have the software installed.
EndNote Training: CDC Library offers training on EndNote on a regular basis – both a basic and advanced course. To view the course descriptions and upcoming training dates, please visit the CDC Library training page .
For assistance with EndNote software, please contact [email protected]
Vendor Support and Services: EndNote – Support and Services (Thomson Reuters) EndNote – Tutorials and Live Online Classes (Thomson Reuters)
Getting Articles:
Articles can be obtained using DocExpress or by searching the electronic journals at the Stephen B. Thacker CDC Library.
IOM Standards for Systematic Reviews: Standard 3.1: Conduct a comprehensive systematic search for evidence
The goal of a systematic review search is to maximize recall and precision while keeping results manageable. Recall (sensitivity) is defined as the number of relevant reports identified divided by the total number of relevant reports in existence. Precision (specificity) is defined as the number of relevant reports identified divided by the total number of reports identified.
Issues to consider when creating a systematic review search:
- All concepts are included in the strategy
- All appropriate subject headings are used
- Appropriate use of explosion
- Appropriate use of subheadings and floating subheadings
- Use of natural language (text words) in addition to controlled vocabulary terms
- Use of appropriate synonyms, acronyms, etc.
- Truncation and spelling variation as appropriate
- Appropriate use of limits such as language, years, etc.
- Field searching, publication type, author, etc.
- Boolean operators used appropriately
- Line errors: when searches are combined using line numbers, be sure the numbers refer to the searches intended
- Check indexing of relevant articles
- Search strategy adapted as needed for multiple databases
- Cochrane Handbook: Searching for Studies See Part 2, Chapter 6
A step-by-step guide to systematically identify all relevant animal studies
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Literature Search: Databases and Gray Literature
The literature search.
- A systematic review search includes a search of databases, gray literature, personal communications, and a handsearch of high impact journals in the related field. See our list of recommended databases and gray literature sources on this page.
- a comprehensive literature search can not be dependent on a single database, nor on bibliographic databases only.
- inclusion of multiple databases helps avoid publication bias (georaphic bias or bias against publication of negative results).
- The Cochrane Collaboration recommends PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) at a minimum.
- NOTE: The Cochrane Collaboration and the IOM recommend that the literature search be conducted by librarians or persons with extensive literature search experience. Please contact the NIH Librarians for assistance with the literature search component of your systematic review.
Cochrane Library
A collection of six databases that contain different types of high-quality, independent evidence to inform healthcare decision-making. Search the Cochrane Central Register of Controlled Trials here.
European database of biomedical and pharmacologic literature.
PubMed comprises more than 21 million citations for biomedical literature from MEDLINE, life science journals, and online books.
Largest abstract and citation database of peer-reviewed literature and quality web sources. Contains conference papers.
Web of Science
World's leading citation databases. Covers over 12,000 of the highest impact journals worldwide, including Open Access journals and over 150,000 conference proceedings. Coverage in the sciences, social sciences, arts, and humanities, with coverage to 1900.
Subject Specific Databases
APA PsycINFO
Over 4.5 million abstracts of peer-reviewed literature in the behavioral and social sciences. Includes conference papers, book chapters, psychological tests, scales and measurement tools.
CINAHL Plus
Comprehensive journal index to nursing and allied health literature, includes books, nursing dissertations, conference proceedings, practice standards and book chapters.
Latin American and Caribbean health sciences literature database
Gray Literature
- Gray Literature is the term for information that falls outside the mainstream of published journal and mongraph literature, not controlled by commercial publishers
- hard to find studies, reports, or dissertations
- conference abstracts or papers
- governmental or private sector research
- clinical trials - ongoing or unpublished
- experts and researchers in the field
- Library catalogs
- Professional association websites
- Google Scholar - Search scholarly literature across many disciplines and sources, including theses, books, abstracts and articles.
- Dissertation Abstracts - dissertation and theses database - NIH Library biomedical librarians can access and search for you.
- NTIS - central resource for government-funded scientific, technical, engineering, and business related information.
- AHRQ - agency for healthcare research and quality
- Open Grey - system for information on grey literature in Europe. Open access to 700,000 references to the grey literature.
- World Health Organization - providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries and monitoring and assessing health trends.
- New York Academy of Medicine Grey Literature Report - a bimonthly publication of The New York Academy of Medicine (NYAM) alerting readers to new gray literature publications in health services research and selected public health topics. NOTE: Discontinued as of Jan 2017, but resources are still accessible.
- Gray Source Index
- OpenDOAR - directory of academic repositories
- International Clinical Trials Registery Platform - from the World Health Organization
- Australian New Zealand Clinical Trials Registry
- Brazilian Clinical Trials Registry
- Chinese Clinical Trial Registry -
- ClinicalTrials.gov - U.S. and international federally and privately supported clinical trials registry and results database
- Clinical Trials Registry - India
- EU clinical Trials Register
- Japan Primary Registries Network
- Pan African Clinical Trials Registry

Your browser is ancient! Upgrade to a different browser to experience this site.
Combines the best of Evidence-Based Health Care , information technologies and a network of experts to provide a unique tool for people making decisions concerning clinical or health-policy questions.
Epistemonikos database in a nutshell
One-stop shop.
All of the evidence relevant for health decision-making, including world's largest systematic review database, curated and annotated by our network of collaborators.
Interconnected evidence
Articles are connected, so you can easily move from any article to all the evidence answering the same question. You can see the forest, not only the trees!
Powerful search engine
Find what you are looking for using our multilingual foolproof search tools. Searching for the best available evidence has never been easier!
Testimonials

...for example, Epistemonikos connects systematic reviews and their included studies, and thus allows clustering of systematic reviews based on the primary studies they have in common. Epistemonikos is also unique in offering an appreciable multilingual user interface, multilingual search, and translation of abstracts in more than 9 languages.
Users' Guides to the Medical Literature

With over 100,000 systematic reviews plus the studies included in those reviews plus overviews of reviews, Epistemonikos is not only the world's largest database of systematic reviews, it is one of the easiest to use. In addition to making it easy to quickly find them, it makes it easy to compare systematic reviews. Epistemonikos has quickly become an essential tool for anyone interested in using evidence to inform healthcare decisions. It also is a unique platform for creating specialised databases, such as PDQ-Evidence , a database of systematic reviews that provides quick access to the best evidence for informing decisions about health systems.
Andy Oxman Research Director, Global Health Unit, Norwegian Institute of Public Health

Epistemonikos has become one of the largest databases of health evidence, a user friendly source that includes over 100,000 systematic reviews and hundreds of thousands of individual studies. Apart from the amount of information, Epistemonikos is unique in a number of ways. First, it caters to non-English speaking users - you can conduct your search in any of 9 languages. Second, a "matrix of evidence" tool facilitates quick searching and updating. The tool identifies all systematic reviews that address a particular question, and all the studies included in those reviews, displaying a cross-tabulation of reviews and studies. It also allows you to stay up to date: after you save your search, any new review will appear automatically.
Gordon Guyatt Distinguished University Professor at McMaster University in Hamilton, Ontario. Pioneer in evidence-based medicine, coined the term "evidence-based medicine" in 1990.

Iain Chalmers Founder of the Cochrane Collaboration 1st Cochrane Lecture, 20th Cochrane Colloquium
A Systematic Review screener - simple and user friendly software to get your screening done without a hassle
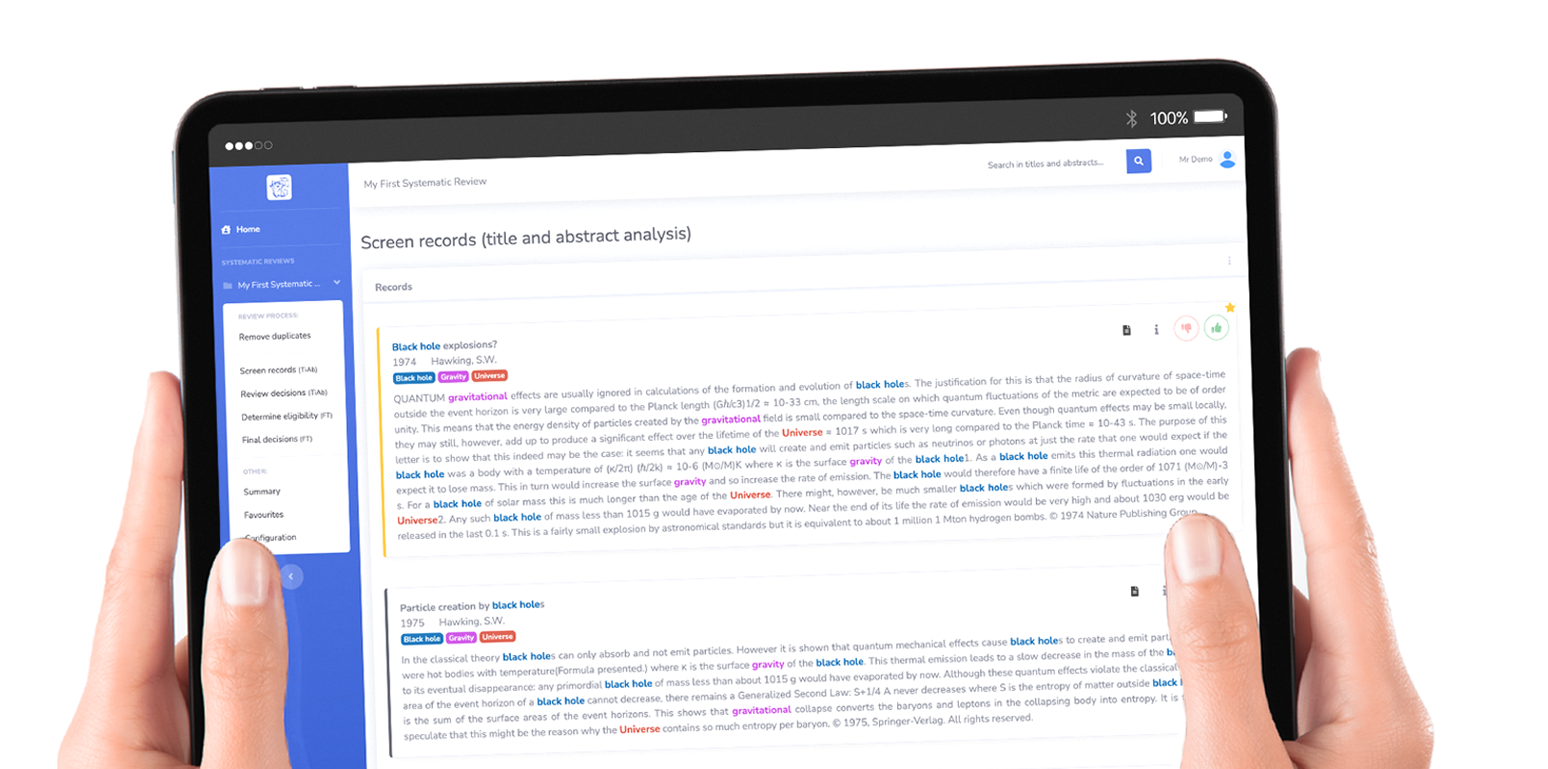
Easing a tedious task
Catchii is a systematic (literature) review (SR) screener designed to provide a good user experience while going through a large dataset of scientific articles. Catchii supports all main stages of an SR, from detecting duplicates to creating PRISMA flowcharts for the manuscript. Screening can be done on your computer, tablet, or smartphone, making it an easy way to get some work done in the office, at home or even on the plane.
Catchii aims to be a fast and user-friendly SR screener, helping you to catch all the papers you are looking for in a short time. In addition to providing all the desirable features of an SR screener, it is also completely free.
Catchii is published on Research Synthesis Methods .
Main features of Catchii
Other features are for you to discover!
Free to use
There are no subscription fees, no trial periods, no paid pro versions, and no ads. There is only one version of Catchii, which is free to use on your computer and mobile. This is a non-profit project for the whole community of researchers.
Multiple user support
At least two reviewers should participate in the screening process. Catchii allows you to add multiple users to a review with different roles. Users can have roles such as a reviewer, reviewer with the right to resolve disagreements or a manager.
Reference importing
The SR process begins with conducting searches in databases. Catchii enables you to easily import references from major online databases such as PubMed, Scopus, Web of Science, OVID (e.g. PsycINFO, MEDLINE) or your reference manager and a CSV file.
Duplicate detection
Before the start of the screening process, duplicate records must be removed. Catchii has a built-in duplicate detection feature that scans through titles and abstracts and provides a list of potential duplicate records (highly similar abstracts and/or titles) for you to review and remove.
Distinct phases
The SR process consists of two phases: title and abstract screening, and full-text analysis. In each stage, a record can be either included or excluded, followed by solving any discrepancies. Screening can be done even without an internet connection by using the Offline screening functionality. Finally, data from included articles can be extracted.
Keyword highlighting
It is much easier to screen records when we can find the keywords we are interested in or those that are part of our exclusion criteria. With the keyword highlighting feature, it is easy and quick to spot the words you are interested in, which helps to speed up your decision-making process. Different groups and colours can be used.
AI screening
Can't find a second reviewer to screen articles or want to assess abstracts in a foreign language that you don't speak? You can use the Robot Reviewer (AI) to screen all records in the first phase, even if they are written in another language. The Robot Reviewer makes decisions for each record along with providing a reason for each decision.
Export to various formats
After the screening process, it's time for data extraction. You may want to export all of your screened and eligible papers into your reference manager program (e.g., EndNote, Mendeley, etc.). No worries, this can be easily done, and you can even attach PDFs. Finally, you can also export the PRISMA flowchart for your manuscript.
Let us know when you have any issues, questions or just want to propose a great addition to the platform!
E-mail (general).
E-mail (Issues)
Send us a message
Terms of service.

IMAGES
COMMENTS
The journal publishes high quality systematic review products including systematic review protocols, systematic reviews related to a very broad definition of human health, (animal studies relevant for human health), rapid reviews, updates of already completed systematic reviews, and methods research related to the science of systematic reviews, such as decision modelling.
Literature searching for a systematic review. IOM Standards for Systematic Reviews: Standard 3.1: Conduct a comprehensive systematic search for evidence. The goal of a systematic review search is to maximize recall and precision while keeping results manageable.
A Cochrane review is a systematic review that attempts to identify, appraise and synthesize evidence to answer a specific research question. Researchers conducting systematic reviews use explicit, systematic methods aimed at minimizing bias, to produce reliable findings to inform decision-making. We encourage authors of Cochrane reviews to ...
Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT) The World's #1 Systematic Review Tool Reviewers
The Cochrane Library is a collection of high-quality, independent evidence to inform healthcare decision-making, including the Cochrane Database of Systematic Reviews and the CENTRAL register of controlled trials.
Time: According to Cochrane, it takes 18 months on average to complete a Systematic Review. The average systematic review from beginning to end requires 18 months of work. "…to find out about a healthcare intervention it is worth searching research literature thoroughly to see if the answer is already known. This may require considerable ...
Please contact the NIH Librarians for assistance with the literature search component of your systematic review. Databases. Cochrane Library. A collection of six databases that contain different types of high-quality, independent evidence to inform healthcare decision-making. Search the Cochrane Central Register of Controlled Trials here. Embase
A systematic review attempts to identify, appraise and synthesize all the empirical evidence that meets pre-specified eligibility criteria to answer a specific research question. Researchers conducting systematic reviews use explicit, systematic methods that are selected with a view aimed at minimizing bias, to produce more reliable findings to ...
Epistemonikos has quickly become an essential tool for anyone interested in using evidence to inform healthcare decisions. With over 100,000 systematic reviews plus the studies included in those reviews plus overviews of reviews, Epistemonikos is not only the world's largest database of systematic reviews, it is one of the easiest to use. In addition to making it easy to quickly find them, it ...
Catchii is a systematic (literature) review (SR) screener designed to provide a good user experience while going through a large dataset of scientific articles. Catchii supports all main stages of an SR, from detecting duplicates to creating PRISMA flowcharts for the manuscript. Screening can be done on your computer, tablet, or smartphone ...