- Case report
- Open access
- Published: 10 August 2020

Non-classical presentation of vitamin D deficiency: a case report
- Mohanad Kamaleldin Mahmoud Ibrahim 1 &
- Mustafa Khidir Mustafa Elnimeiri 2
Journal of Medical Case Reports volume 14 , Article number: 126 ( 2020 ) Cite this article
7963 Accesses
22 Altmetric
Metrics details
Vitamin D is a fat-soluble vitamin; vitamin D is essential to sustain health and it protects against osteoporosis. It is crucial to the human body’s physiology in terms of muscular movement and neurological signal transmission, and to the immune system in defense against invading pathogens.
Case presentation
This was a case of a 26-year-old Sudanese woman who presented with a 2-year history of anosmia, recurrent nasal polyps, back pain, and chronic fatigue. She was diagnosed as having a case of vitamin D deficiency and responded well to treatment.
There is an association between vitamin D deficiency and recurrent allergic nasal conditions.
Peer Review reports
Vitamin D is a fat-soluble vitamin; it is naturally present in some foods and as dietary supplements. It is also produced endogenously through exposure to ultraviolet rays from sunlight. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation. The first occurs in the liver and produces 25-hydroxyvitamin D (25(OH)D), also known as calcidiol. The second occurs in the kidney and forms the physiologically active 1,25-dihydroxy vitamin D (1,25(OH) 2 D), also known as calcitriol [ 1 ].
Vitamin D is found in cells throughout the body; vitamin D is essential to sustain health and it protects against osteoporosis. It is crucial to the human body’s physiology in terms of muscular movement and neurological signal transmission, and to the immune system in defense against invading pathogens [ 2 ].
Although there are different methods and criteria for defining vitamin D levels, the criteria Holick proposed have been widely accepted. In this proposal, vitamin D deficiency is defined as blood level of less than 20 ng/ml; insufficiency of vitamin D is defined as blood levels ranging between 20 and 29.9 ng/ml and sufficiency if greater than or equal to 30 ng/ml [ 3 ]. About one billion people globally have vitamin D deficiency and 50% of the population has vitamin D insufficiency. The majority of affected people with vitamin D deficiency are the elderly, obese patients, nursing home residents, and hospitalized patients. Vitamin D deficiency arises from multiple causes including inadequate dietary intake and inadequate exposure to sunlight. Certain malabsorption syndromes such as celiac disease, short bowel syndrome, gastric bypass, some medications and cystic fibrosis may also lead to vitamin D deficiency [ 4 ].
Vitamin D deficiency is now more prevalent than ever and should be screened in high-risk populations. Many conflicting studies now show an association between vitamin D deficiency and cancer, cardiovascular disease, diabetes, autoimmune diseases, and neuropsychiatric disorders [ 5 , 6 ].
This was a case of a 26-year-old Sudanese woman, married, who has a 3-year-old boy. This woman presented to our ear, nose, and throat (ENT) department complaining of anosmia for the past 2 years. She had a history of two functional endoscopic sinus surgeries (FESSs) for nasal polyps: the first one was 6 years ago and the second one was 3 years prior to presentation. She complained of being highly sensitive to different irritants including dust, weather change, perfumes, and pets.She also stated that she attended more than three different physicians due to generalized fatigue and getting tired easily after simple daily activity in addition to sleeping for more than 10 hours a day.She attended an orthopedic clinic for unspecified lower back pain that was not related to any type of trauma or physical activity; a lumbosacral magnetic resonance imaging (MRI) was done and revealed no abnormal findings.She mentioned that she is known to be anxious most of the time and aggressive toward simple reactions from her family members. She had no psychiatric history and was not using any medications.
She was not known to be diabetic or hypertensive or to have any chronic illnesses; she was not on any regular medication. She is a housewife of high socioeconomic status; she is well educated, graduated from dental school with a bachelor’s degree, but currently not employed. She has never consumed tobacco or alcohol; she practiced regular cardio exercises.On examination, she looked healthy, well, not pale or jaundiced. Her pulse rate was 74/minute and her blood pressure was 118/70. Her body mass index (BMI) was 26.8. All systems examinations were normal except for bilateral nasal polyps. Complete blood count (CBC), renal function test (REF), electrolyte, liver function test (LFT), thyroid function test (TFT), urine analysis (general urine test), antinuclear antibody (ANA), and rheumatoid factor (RF) were all normal. An imaging profile included lumbo-sacral MRI, a computed tomography (CT) scan of her sinuses, and electrocardiogram (ECG), which were normal except for bilateral nasal polyps and severe sinusitis that looked allergic to fungi in nature.She underwent FESS surgery to remove the polyps and clean out her sinuses; up to 6 weeks after surgery she used nasal steroids (mometasone furoate 0.005%) two times a day, but her symptoms regarding anosmia were not improved. MRI of her brain and a CT scan of her sinuses were done and both revealed normal features. A vitamin D deficiency was suggested and the laboratory results revealed a low vitamin D level of 7 ng/ml. Treatment with vitamin D supplement was prescribed at 50,000 international units (IU) weekly for 8 weeks and then 1000 IU maintenance dose daily, she was advised to take food rich in vitamin D and get exposed to sunlight for 20 minutes three times a week after the loading dose of supplement. She was at regular follow-up for 6 months; at rates of weekly for the first month, every 2 weeks for the second month, and monthly for the rest of the follow-up period. At each visit, she was assessed with clinical history and examination. It was noticed that the symptoms of tiredness, sleeping, anosmia, and back pain were dramatically improving during that period. At the 6 months follow-up, her blood level of vitamin D was normal, she described her condition as free from all symptoms, and she returned back to normal physical activity.
Discussion and conclusions
This was a non-classical case of vitamin D deficiency of a 26-year-old woman who presented with chronic anosmia and recurrent nasal polyps. She was diagnosed as having a case of vitamin D deficiency and responded well to vitamin D replacement therapy. This case correlated an association between decreased levels of vitamin D and recurrent nasal polyps that led in time to chronic anosmia as a result of chronic high sensitivity reactions triggered by our patient’s autoimmune system. The literature links chronic rhinosinusitis with nasal polyps (CRSwNP) with asthma and allergic rhinitis, but the cellular and molecular mechanisms that contribute to the clinical symptoms are not fully understood. Sinonasal epithelial cell barrier defects, increased exposure to pathogenic and colonized bacteria, and dysregulation of the host immune system are all thought to play prominent roles in disease pathogenesis [ 7 ].
Despite all the previous surgical and medical interventions over the past 6 years, our patient’s condition did not improve and she still complained of anosmia. A study revealed that this patient was experiencing excessive allergic reactions that led to recurrent nasal polyps. It is well known that classical clinical effects of vitamin D deficiency are bones and musculoskeletal-related disorders, several lines of evidence demonstrate the effects of vitamin D on pro-inflammatory cytokines, regulatory T cells, and immune responses, with a conflicting interpretation of the effects of vitamin D on allergic diseases [ 8 ].
The working diagnosis was suggested in relation to some musculoskeletal symptoms and chronic fatigue especially when the imaging profile for her lower back and all routine investigations were normal. It has been suggested that clinicians should routinely test for hypovitaminosis D in patients with musculoskeletal symptoms, such as bone pain, myalgias, and generalized weakness which might be misdiagnosed as fibromyalgia and chronic fatigue [ 9 ]. The most common causes of anosmia were assessed as well and they were negative, these included sinonasal diseases, post infectious disorder, and post-traumatic disorder, and congenital defects and disorders caused by neurodegenerative disease [ 10 ].
Thus blood level for vitamin D was requested and the results were of low D level.
In the past history of the previous nasal polyps surgeries, our patient noted that there was no anosmia and her main complaints were classic complaints of sinusitis, including sneezing, nasal blockage and headache. Soon after surgery her symptoms improved except for the allergy-related symptoms, despite usage of inhaled steroids spray. She stated that, at the last time, the presentation was different since it was only anosmia, indicating that there was significant inflammation that affected the smell receptors around the olfactory epithelium. After the last nasal polyps and sinuses drainage surgery, the symptoms related to allergic reactions, including chronic sneezing, did not improve for up to 6 weeks and she was still suffering from hyposmia, although that was a fair postoperative period for recovery.
The symptoms of anosmia and sneezing, and other systematic symptoms, gradually started to improve after vitamin D supplements, indicating that the main reason behind her symptoms was vitamin D deficiency. She was followed up for up to 6 months after establishment of vitamin D supplements and at the last follow-up she had a normal sense of smell, and she was free from back pain, fatigue, and allergy-related symptoms.
This was a non-classical presentation as our patient was young and she did not have alkaline phosphatase, calcium, and phosphorus abnormalities [ 11 ] that are expected in cases of vitamin D deficiency.
This case revealed an association between decreased levels of vitamin D and recurrent nasal polyps that led to anosmia as a result of hypersensitive reactions produced by the body’s systems.
Although vitamin D deficiency is prevalent, measurement of serum 25(OH)D level is expensive, and universal screening is not supported. However, vitamin D testing may benefit those at risk for severe deficiency.
It is highly recommended to consider vitamin D deficiency among all patients with unspecified symptoms or in cases of non-diagnosed disorder regardless of the presenting complaint.
In conclusion, there is an association between vitamin D deficiency and recurrent allergic nasal conditions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic rhinosinusitis with nasal polyps
Computed tomography
Electrocardiogram
Ear, nose, and throat
Functional endoscopic sinus surgery
Body mass index
Complete blood count
Renal function test
Liver function test
Thyroid function test
Antinuclear antibody
Rheumatoid factor
International unit
National Institutes of Health. Vitamin D: fact sheet for health professionals. 2020. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#en2 . Accessed 10 Apr 2020.
Google Scholar
National Institutes for Health (NIH). Vitamin D fact sheet for consumers. 2019. https://ods.od.nih.gov/factsheets/VitaminD-Consumer/ . Accessed 20 Dec 2019.
Kuriacose R, Olive KE. Vitamin D insufficiency/deficiency management. South Med J. 2014;107(2):66–70. https://doi.org/10.1097/SMJ.0000000000000051 .
Article CAS PubMed Google Scholar
Sizar O, Khare S, Givler A. Vitamin D deficiency. Treasure Island: Stat Pearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK532266/ . PMID: 30335299. Accessed 20 Dec 2019.
Wlliam B, Fatme A, Meis M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D 3 supplementation can have important patient and public health benefits. Eur J Clin Nutr. 2020;74:366–76. https://doi.org/10.1038/s41430-020-0564-0 .
Article CAS Google Scholar
Hanmin W, Weiwen C, Dongqing L, Xiaoe Y, Xiaode Z, Nancy O, et al. Vitamin D and chronic diseases. Aging Dis. 2017;8(3):346–53. https://doi.org/10.14336/AD.2016.1021 .
Article Google Scholar
Whitney W, Ropert P, Robert C. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(04):565–72. https://doi.org/10.1016/j.jaip.2016.04.012 .
Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. https://doi.org/10.4065/mcp.2010.0567 .
Article CAS PubMed PubMed Central Google Scholar
Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752–8. https://doi.org/10.4065/mcp.2010.0138 .
Article PubMed PubMed Central Google Scholar
Sanne B, Elbrich M, Duncan B, Antje W, Veronika S, Joel D, et al. Anosmia: a clinical review. Chem Senses. 2017;42(7):513–23. https://doi.org/10.1093/chemse/bjx025 .
Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis. BMJ Case Rep. 2014; https://doi.org/10.1136/bcr-2014-204558 .
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Community Medicine and Epidemiology, Faculty of Medicine, Ibn Sina University, Khartoum, Sudan
Mohanad Kamaleldin Mahmoud Ibrahim
Preventive Medicine and Epidemiology, Alneelain University, Khartoum, Sudan
Mustafa Khidir Mustafa Elnimeiri
You can also search for this author in PubMed Google Scholar
Contributions
MI analyzed and interpreted the findings of the case report and was the major contributor in writing the manuscript. ME reviewed the report and added valuable comments. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Mohanad Kamaleldin Mahmoud Ibrahim .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval was obtained from Albasar Institutional Review Board.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ibrahim, M.K.M., Elnimeiri, M.K.M. Non-classical presentation of vitamin D deficiency: a case report. J Med Case Reports 14 , 126 (2020). https://doi.org/10.1186/s13256-020-02454-1
Download citation
Received : 26 March 2020
Accepted : 08 July 2020
Published : 10 August 2020
DOI : https://doi.org/10.1186/s13256-020-02454-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vitamin D deficiency
- Nasal polyps
- Chronic fatigability
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Case Report
- Open access
- Published: 14 May 2009
Nutritional vitamin D deficiency: a case report
- Rachel L Stevens 1 &
- Corey Lyon 1
Cases Journal volume 2 , Article number: 7000 ( 2009 ) Cite this article
12k Accesses
3 Citations
Metrics details
We present a 6-month-old African American male child with a chief complaint of failure to appropriately gain weight despite adequate caloric intake via breastfeeding. While he has met developmental milestones he appears small for age and is diagnosed with failure to thrive after crossing two major growth curve percentiles. After appropriate diagnostic workup, a diagnosis of nutritional vitamin D deficiency (rickets) was reached and supplementation was initiated with ensuing adequate catch-up growth.
Introduction
Rickets is often considered an "old" disease, a nutritional deficiency that has plagued communities for centuries. The re-emergence of vitamin D deficiency in westernized societies is thought to be multifactorial secondary to poor dietary intake, popularization of breastfeeding, and diminished exposure to sunlight. It is the most common metabolic bone disease in the world and is easily treatable as well as preventable with sun exposure and dietary supplementation [ 1 ].
Vitamin D is a prohormone essential for absorption of calcium from the intestines. Its supply stems from two well-known sources: exposure to sunlight and dietary intake, which accounts for less than 10% [ 2 ]. Vitamin D is primarily made in the skin after exposure to UV-B radiation (290 to 315 nm wavelengths) [ 2 ]. In rickets, decreased stores of this prohormone leads to low levels of ionized calcium, which initially stimulates parathyroid hormone release to initiate calcium resorption in the renal tubules (along with loss of phosphorous), and increase 1,25 dihydroxy vitamin D synthesis. A level of 25 hydroxy vitamin D (25-OH D) less than 12.5 nmol/L (5 ng/mL) is suggested for the diagnosis of rickets with a healthy maintenance level of approximately greater than 50 nmol/L (20 ng/mL) [ 1 , 2 ]. It should be noted that newer data suggests a lower limit of 80 nmol/L may be a more acceptable level in adults [ 1 , 2 ].
Case presentation
We present a 6-month-old African American male child with poor interval growth. His mother has noticed that though he is thought to be breastfeeding appropriately, as defined by feeding 4 ounces of pumped breast milk every 2-3 hours, and has been meeting developmental milestones, his weight and height are not as expected. He has been exclusively breastfed and his mother has not introduced solid foods as of yet to his diet.
He was a full term, spontaneous vaginal delivery without complications during the pregnancy or labor. He was in the 50 th percentile for both height and weight at his 2 month visit, but has fallen to below the 3 rd percentile for weight and is at the 3 rd percentile for height. He is on no medications, there are no other siblings with failure to thrive and his mother has no post-partum depression or substance abuse issues. There is no family history of malabsorptive conditions. His mother and father are of normal stature.
His review of systems is negative for emesis, diarrhea, fever, appetite changes, swallowing abnormalities, respiratory symptoms, apnea, repeated acute illnesses, or frequent injuries.
His weight at the four month well child visit was 6477 grams with a length of 63.5 cm increasing to only 6761 grams and a length of 66 cm by his six month well child visit. His vitals signs are otherwise stable. His physical exam is significant for an alert, playful, developmentally appropriate child, small for his age. His head/neck, cardiac, respiratory, gastrointestinal, genitourinary, musculoskeletal and neurological exams were within normal limits.
He is appropriately diagnosed with failure to thrive at this visit based on deviation across two major percentiles on standardized growth curves. His estimated weight needs were calculated to 0.33 kg/month and a follow up visit was established in one month with addition of solid foods and continued breastfeeding with the addition of formula to pumped breast milk for increased caloric intake. His mother was also instructed to keep a strict food diary.
Interval weight gain was not maintained with a weight of 7045 grams at follow up despite adequate caloric intake estimated based on his food diary and formula supplementation. Laboratory studies ordered were complete metabolic profile, thyroid stimulating hormone (TSH), lead level, and complete blood count (CBC). Electrolytes, kidney function, bilirubin, AST, ALT, protein, albumin, TSH, CBC, and lead were all normal. Alkaline phosphatase was elevated at 4280 (on repeat 6310). Normal should be less than 500 IU/L in neonates and 1000 IU/L in children up to age 9. Follow-up labs including gamma glutamyl transferase (which was normal, suggestive of boney resorption) [ 2 ], C reactive protein, T3, free T4, phosphate, parathyroid hormone, and 25-OH D were ordered. Phosphate was low at 2.9 (normal 3.0-4.5) and the vitamin D level was 11 (45-50 ng/mL).
A skeletal survey was ordered showing metaphyseal fraying and cupping of bilateral distal femurs, bilateral proximal and distal tibiae and fibulae, bilateral proximal and distal humeri, bilateral distal radii and ulni and the distal aspects of 2 nd through the 5 th metacarpals most consistent with rickets of the extremities, see radiograph 1 and 2. These classic findings may be paired with a separation of the periosteum from the diaphysis secondary to unmineralized osteoid when evaluating radiographic evidence of rickets [ 1 , 3 ]. Radiographic improvement should manifest within 3 months of appropriate treatment. Underlying malabsorptive conditions or noncompliance should be considered if this is not observed [ 2 ].
The diagnosis of rickets was made and the patient was started on 2000 IU of vitamin D and calcium carbonate 1000 mg daily. He was also started on iron sulfate 22 mg daily and Zinc 20 mg daily as recommended by pediatric endocrinology and nutrition staff. His catch-up growth has lead to a current weight (at 2 years of age) in the 45 th percentile and height in the 30 th percentile (Figure 1 ). Follow up labs (calcium, phosphorous, alkaline phosphatase) should be performed one month after therapy is initiated and again at 3 months along with magnesium, PTH, and 25-OH D [ 1 , 2 ]. Follow up labs in this patient indicated an improvement in vitamin D up to 29 ng/mL with ongoing supplementation continuing.
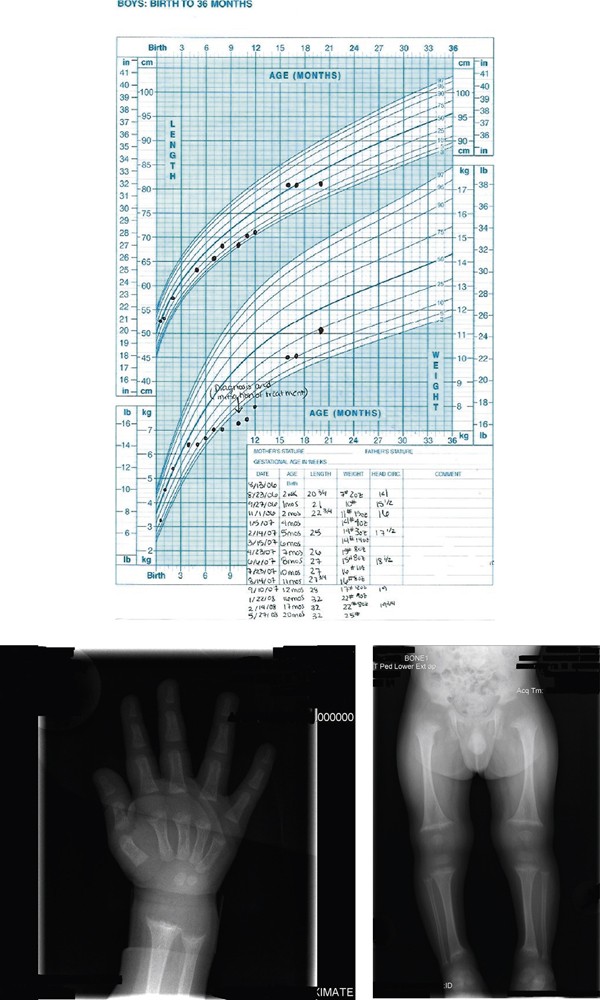
Growth Chart: Growth chart from birth to 20 months showing failure of appropriate growth and then his catch up growth after appropriate treatment . Radiograph Wrist/Hand: Metaphyseal fraying and cupping of distal radius and ulna and the distal aspects of the second through fifth metacarpals. Skeletal survey: Metaphyseal fraying and cupping involving bilateral distal femurs, bilateral proximal and distal tibiae and fibulae, bilateral proximal and distal humeri.
Infants and adolescents are predisposed to rickets secondary to increased flux in body composition and rates of rapid bone growth causing increased need/utilization of calcium and phosphate. Further increased risk is associated with dark-skinned individuals, lack of UV-B exposure, solely breastfed infants and prematurity. Screening should be considered for children with poor growth/development, seizure activity/tetany, and children with chronic malabsorptive states.
It is indisputable that breast milk is the ideal nutrition for infants, however, it only contains 15 - 50 IU/L of vitamin D [ 1 , 2 , 4 ]. There is limited prevalence estimates for vitamin D deficiency rickets in North America and the United Kingdom. Reported and published cases in the United States increased from 65 between 1975 to 1985 to 228 from 1986 to present [ 2 ]. This has led for the American Academy of Pediatrics (AAP) recommendation of vitamin D supplementation in all breast fed infants who do not consume 500 mL/day of vitamin D fortified formula and all non-breastfed infants that do not consume at least 500 mL/day of vitamin D fortified formula. It is recommended this begin in the first days of life and continue through childhood/adolescence [ 2 ]. The 2003 AAP breastfeeding guidelines suggest 200 IU/day to maintain a minimum level of 27.5 nmol/L, however this has been shown insufficient for prevention of all cases of rickets leading to controversy and newer recommendations for 400 IU/day, especially in deeply pigmented breastfed infants [ 2 , 5 ]. Despite appropriate breastfeeding technique and the appropriate amount of breast milk our patient was at risk for vitamin D deficiency secondary to the lack of oral vitamin D supplementation and his ethnicity.
The primary source of vitamin D (sunlight) is dependent on geographic location as well as outdoor exposure. To maintain a low normal level (>27.5 nmol/L) of vitamin D, a fully clothed child would have to spend two hours outside weekly and darker skinned individuals may require exposures up to 6-10 times this amount [ 1 , 2 ]. Sunscreen with an SPF of 15 reduces synthetic capacity by up to 98% [ 2 , 6 ]. The current AAP recommendation (to prevent sunburns and reduce skin cancer risk) is to keep infants less than 6 months of age out of direct sunlight and encourage the use of protective clothing/sunscreen again increasing the risk of vitamin D deficiency in this patient [ 2 ].
Because of this recommendation, management of vitamin D deficiency is via oral vitamin supplementation. Ergocalciferol (plant formulated vitamin D2) or Cholecalciferol (animal formulated vitamin D3) at >5000 IU daily for 2-4 months is suggested for toddlers greater than 12 months of age and up to 10,000 IU in adolescents. In younger infants (1-12 months) 1,000-5000 IU daily has been suggested. In infants <1 month old, 1000 IU daily is recommended [ 2 ]. Once laboratory values have normalized, maintenance of 400 IU per day is suggested [ 1 , 2 ]. If compliance is a concern a one time treatment with high dose oral formulation is appropriate (100,000-600,000 IU) over 1-5 days with a follow up dose in 3 months if necessary [ 2 ]. It is suggested that vitamin D3 may be up to 3 times as potent as D2 and may be preferable, especially when used in bolus form. Some formulations of vitamin D may contain propylene glycol, which is toxic at high doses so caution is advised [ 2 ].
Conclusions
While rickets is a disease that has plagued society for many centuries it is certainly still a significant cause for both skeletal and non-skeletal complications in today's culture. Despite recommendations for supplementation in all breast-fed infants and fortification of multiple household food items, it is still a relatively common nutritional deficiency. Education on proper nutrition during pregnancy and supplementation during breastfeeding is necessary to prevent its growing resurgence. Proper childhood maintenance visits with growth and development screenings are critical for early detection of this easily treatable condition.
Written informed consent was obtained from the patient's mother for publication of this case report and accompanying images of radiographs and growth chart. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
American Academy of Pediatrics
Alanine Aminotransferase
Aspartate Aminotransferase
Complete blood count
Parathyroid hormone
Sun Protection Factor
Thyroid stimulating hormone
Ultra violet-B.
Dimitri P, Bishop N, Rickets : New insights into a re-emerging problem. Curr Op in Ortho. 2007, 18: 486-493. 10.1097/BCO.0b013e3282b97118.
Article Google Scholar
Misra M, Pacaud D, Petryk A, et al: Vitamin D deficiency and its management: review of current knowledge and recommendations. Pediatrics. 2008, 122: 398-417. 10.1542/peds.2007-1894.
Article PubMed Google Scholar
Hickey L, Cross C, Ewald MB: Nutritional rickets: beyond the chief complaint. Ped Emer Care. 2006, 22: 121-123. 10.1097/01.pec.0000199559.96356.3d.
Ward LM, et al: Vitamin D deficiency rickets among children in Canada. CMAJ-JAMC. 2007, 177: 161-166.
Breastfeeding and the Use of Human Milk. Pediatrics. 2005, 115: 496-506. 10.1542/peds.2004-2491.
Hickey L, Gordon CM: Vitamin D Deficiency: new perspective on an old disease. Curr Op in Endo and Diab. 2004, 11: 18-25. 10.1097/00060793-200402000-00006.
Article CAS Google Scholar
Download references
Author information
Authors and affiliations.
Research Family Medicine Residency, Research Medical Center, 6650 Troost Ave, Kansas City, 64131, USA
Rachel L Stevens & Corey Lyon
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Corey Lyon .
Additional information
Competing interests.
The author(s) declare that they have no competing interests.
Authors' contributions
RS provided care for this patient when he presented to her clinic at 6 months of age. RS completed the work-up, researched and initiated the treatment. CL provided assistance with the preparation of the manuscript

Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Stevens, R.L., Lyon, C. Nutritional vitamin D deficiency: a case report. Cases Journal 2 , 7000 (2009). https://doi.org/10.1186/1757-1626-2-7000
Download citation
Received : 02 January 2009
Accepted : 03 March 2009
Published : 14 May 2009
DOI : https://doi.org/10.1186/1757-1626-2-7000
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Thyroid Stimulate Hormone
- Nutritional Vitamin
- Adequate Caloric Intake
- Parathyroid Hormone Release
Cases Journal
ISSN: 1757-1626
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis
Kiyoshi shikino, masatomi ikusaka, tomoko yamashita.
- Author information
- Article notes
- Copyright and License information
Correspondence to Dr Kiyoshi Shikino, [email protected]
Series information
Case Report
Accepted 2014 Jun 6; Collection date 2014.
A 34-year-old Japanese woman presented with a 2-year history of generalised bone pain, muscle weakness and gait disturbance. The patient had been following a restricted diet (without fish or dairy products) and avoiding ultraviolet exposure for 8 years to manage her worsening atopic dermatitis. Physical examination revealed generalised bone tenderness and bilateral symmetric proximal muscle weakness. Vitamin D-deficient osteomalacia was diagnosed based on the laboratory examination findings, which indicated high serum alkaline phosphatase, high intact parathyroid hormone, and low 25-hydroxyvitamin D levels. Her symptoms improved after oral active vitamin D and calcium administration. To the best our knowledge, this case is the first report of vitamin D-deficient osteomalacia in an adult patient due to excessive dietary restriction for managing atopic dermatitis. We emphasise the importance of increasing awareness of vitamin D deficiency as a risk factor for the development of osteomalacia, and caution against excessive avoidance of sun exposure and dietary restriction.
Osteomalacia is a metabolic bone disorder characterised by the decreased mineralisation of newly formed osteoid at sites of bone turnover. 1 The clinical symptoms associated with osteomalacia include progressive generalised bone pain, muscle weakness and gait disturbance. 2
Although vitamin D deficiency is among the most common causes of osteomalacia, there is no case report of vitamin D-deficient osteomalacia due to excessive self-restriction for managing atopic dermatitis. In the present report, we describe a case of osteomalacia caused by vitamin D deficiency in an adult patient who strictly complied with dietary restrictions and avoidance of sun exposure to manage her worsening atopic dermatitis.
Case presentation
A 34-year-old Japanese woman was referred to our department with a 2-year history of slowly progressive generalised bone pain, muscle weakness and gait disturbance. The generalised bone pain—especially in the lower back, pelvis and legs—was aggravated by loading. The patient had been following a restricted diet (exclusion of fish and dairy products), and had been avoiding ultraviolet exposure for 8 years, which was part of the management plan prescribed by her dermatologist for worsening atopic dermatitis. The patient had no reported digestive symptoms that would have suggested an absorption disorder. She had been receiving topical steroids for her atopic dermatitis. Her family and personal histories were unremarkable. Physical examination revealed the following normal findings: body temperature, 36.3°C; blood pressure, 110/72 mm Hg; pulse rate, 88 bpm (regular); and respiration rate, 12 breaths/min. However, the examination also indicated generalised bone tenderness with associated indirect pain, bilateral symmetric proximal muscle weakness and a waddling gait.
Investigations
Laboratory examination revealed high serum alkaline phosphatase (1345 U/L (bone type, 71.2%)), high intact parathyroid hormone (511 pg/mL), low serum calcium (7.6 mg/dL), low phosphorus (2.2 mg/dL) and low 25-hydroxyvitamin D (<5 ng/mL, RIA) levels. Liver, pancreas and renal functions were normal. The bone mineral density (BMD) T-score, as measured by dual-energy X-ray absorptiometry, was –5.7 at the lumbar spine and −5.8 at the femoral neck, indicating a low BMD for her chronological age. Moreover, increased bone turnover markers for bone resorption (deoxypyridnoline, 31.2 nmol/mmol Cr (normal range, 2.8–7.6 nmol/mmol Cr)) and formation (osteocalcin, 7.8 ng/mL (normal range, ≤7.0 ng/mL); urinary type\xE2\x85collagen cross-linked N-telopeptide, 2938 nmol BCE/mmol Cr (normal range, 9.3–54.3 nmol BCE/mmol Cr)) were noted.
Arterial blood gas analysis revealed mild metabolic acidosis (pH, 7.37; partial pressure of carbon dioxide, 23.2 mm Hg; partial pressure of oxygen, 98 mm Hg; and bicarbonate, 22.3 mmol/L). The urine analysis results were as follows: protein, negative; glucose, negative; pH, 6; phosphorus, 0.19 g/day; and calcium, 29.3 mg/day. The tubular reabsorption of filtered phosphorus (%TRP, 94%; normal range, 85–95%) and the maximum tubular reabsorptive rate of phosphate (tubular maximum phosphate reabsorption/glomerular filtration rate, 2.93 mg/dL; normal range 2.6–4.4 mg/dL) were within the normal range. Immunoelectrophoretic studies did not show any monoclonal protein in the serum or any Bence-Jones protein in the urine. Anti-SS-A/SS-B antibody tests yielded negative results.
Plain film radiography revealed a pseudofracture in the left inferior pubic rami ( figure 1 ). Fluorodeoxyglucose-positron emission tomography images showed no tumour lesions or abnormal uptake.
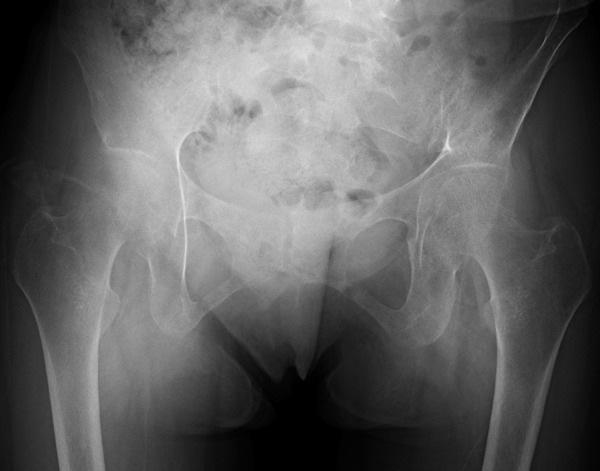
Plain film radiography revealing pseudofracture in the left inferior public rami.
Thus, the patient was diagnosed with osteomalacia due to vitamin D deficiency, which was attributed to the restriction of vitamin D intake and sun exposure avoidance for the management of atopic dermatitis.
Differential diagnosis
Although the main cause of vitamin D deficiency is impairment of endogenous vitamin D 3 synthesis due to the lack of ultraviolet exposure and dietary vitamin D deficiency, this condition is also manifested in cases of malabsorption syndrome due to gastrectomy, intestinal diseases, hepatobiliary diseases and pancreatic insufficiency. 3 4 However, the patient's medical history and laboratory examination results were not suggestive of these conditions.
Hypophosphatemia—a condition in which osteomalacia is caused by impaired phosphorus absorption and reabsorption—is known to be caused by tumour-induced osteomalacia (TIO) and renal tubular acidosis. 5 6 The FGF23 marker is useful for the diagnosis of TIO, as its levels are high in TIO patients and rapidly decrease after resection of the tumours responsible for the TIO. 5 In our patient, the FGF23 level was within the normal range (24 pg/mL), and the presence of apparent tumour lesions was also ruled out by positron emission tomography-CT. Although our patient had mild metabolic acidosis, the urinary findings suggest that it is unlikely to have been caused by renal tubular acidosis. Moreover, the medical history and laboratory examination results did not reveal any findings suggestive of Sjogren syndrome, multiple myeloma or nephrotic syndrome.
Oral administration of active vitamin D (0.75 μg/day) and calcium (1 g/day) was initiated.
Outcome and follow-up
At the 12-month follow- up, the pain, muscle weakness and gait disturbance had been alleviated. Moreover, the serum levels of 25-hydroxyvitamin D, parathyroid hormone, alkaline phosphatase, calcium and phosphorus had improved, with no recurrence of the condition.
Vitamin D plays an important role in bone mineralisation. 7 Thus, the deficiency of vitamin D causes secondary hyperparathyroidism and increased bone turnover, which may eventually result in osteomalacia. 8
The National Health and Nutrition Examination Survey (2001–2004) reported that vitamin D deficiency, including asymptomatic cases, occurs in approximately 6% of the population in the USA and is considered a relatively common disease even in developed nations. 9
The amount of ultraviolet skin exposure in ordinary life contributes to the production of 90–95% of the body's vitamin D requirement. It has been reported that sun exposure at approximately one-fourth the minimal erythema dose to at least one-fourth the total skin area of the body is equivalent to an intake of approximately 250 μg of vitamin D 3 , 10 11 which fully satisfies the daily required intake of 15 μg. 12 In cutaneous synthesis, vitamin D is synthesised through the effects of ultraviolet-B light, which has a wavelength of 280–320 nm and is also known to be able to cause sunburn. 13 Thus, people who avoid sun exposure or use sunscreen that blocks ultraviolet-B may have a higher risk of vitamin D deficiency, as observed in the present case.
In patients who receive an average dietary intake of vitamin D, the occurrence of osteomalacia as a result of vitamin D deficiency is unlikely. However, extremely unbalanced and restricted diets can cause a significantly impaired dietary intake of vitamin D, and may thus result in osteomalacia. For instance, diets deficient in foods containing large amounts of vitamin D such as fish, dairy products and mushrooms, 14 can lead to vitamin D deficiency—this was a pertinent cause of our patient's vitamin D deficiency.
There are occasional case reports stating that paediatric patients with atopic dermatitis developed rickets as a result of vitamin D deficiency caused by inadequate dietary intake. 15–18 To the best of our knowledge, the present case is the first report of an adult patient with osteomalacia associated with atopic dermatitis. Although the treatment for atopic dermatitis includes ultraviolet light therapy, 19 sun exposure is also considered to be an exacerbating factor for atopic dermatitis. 20 In the present case, the excessive avoidance of sun exposure is assumed to be a major factor for the development of osteomalacia caused by vitamin D deficiency. Furthermore, inadequate intake of foods containing vitamin D due to dietary restrictions also promoted the development of osteomalacia. In this case, it was assumed that complete elimination of food allergens and avoidance of sun exposure, to prevent worsening of the atopic dermatitis, caused the vitamin D deficiency, resulting in osteomalacia.
Moreover, other factors such as excessive orientation towards a healthy lifestyle and natural foods and avoidance of ultraviolet exposure in our modern lifestyle can contribute to the development of osteomalacia. Therefore, a possible increase in the prevalence of vitamin D deficiency in the future remains a concern. Thus, this case report highlights the importance of increasing awareness of vitamin D deficiency as a risk factor for the development of osteomalacia, and cautions against excessive avoidance of sun exposure and dietary restrictions.
Learning points.
Vitamin D deficiency is among the most common causes of osteomalacia.
Vitamin D deficiency is considered a relatively common disease even in developed nations.
Excessive orientation towards a healthy lifestyle and natural foods and avoidance of ultraviolet exposure in our modern lifestyle can contribute to vitamin D deficiency.
Awareness of vitamin D deficiency as a risk factor for osteomalacia, and its association with excessive avoidance of sun exposure and dietary restrictions are important.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
- 1. Gifre L, Peris P, Monegal A, et al. Osteomalacia revisited: a report on 28 cases. Clin Rheumatol 2011;30:639–45 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011;86:50–60 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Holick MF, Chen TC. Vitamin D deficiency. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S–6S [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Dibble JB, Sheridan P, Losowsky MS. A survey of vitamin D deficiency in gastrointestinal and liver disorders. Q J Med 1984;53:119–34 [ PubMed ] [ Google Scholar ]
- 5. Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 2002;87:4957–60 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hirukawa M, Funakoshi H, Tsukamoto T, et al. Osteomalacia due to a bladder reconstruction performed 35 years previously. Intern Med 2012;51:2051–5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 2011;364:248–54 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. LeBoff MS, Kohlmeier L, Hurwitz S, et al. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999;281:1505–11 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169:626–32 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Holick MF. Vitamin D requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int 1998;8:S24–9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80:1678S–88S [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Ross AC, Taylor CL, Yaktine AL, et al. Institude of medicine: dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US), 2011 [ PubMed ] [ Google Scholar ]
- 13. Bogh MK. Vitamin D production after UVB: aspects of UV-related and personal factors. Scand J Clin Lab Invest Suppl 2012;243:24–31 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Nakamura K. Vitamin D insufficiency in Japanese populations from the viewpoint of the prevention of osteoporosis. J Bone Miner Metab 2006;24:1–6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Huang LT, Yang W, Wu CL. Vitamin D deficiency rickets due to inappropriate feeding: report of one case. Acta Paediatr Taiwan 2000;41:151–4 [ PubMed ] [ Google Scholar ]
- 16. Fox AT, Du Toit G, Lang A, et al. Food allergy as a risk factor for nutritional rickets. Pediatr Allergy Immunol 2004;15:566–9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. George D, Wasko C, Metry D. Atopic dermatitis and nutritional rickets: an exercise in parental counseling. Pediatr Dermatol 2006;23:102–3 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Yu JW, Pekeles G, Legault L, et al. Milk allergy and vitamin D deficiency rickets: a common disorder associated with an uncommon disease. Ann Allergy Asthma Immunol 2006;96:615–19 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Reynolds NJ, Franklin V, Gray JC, et al. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357:2012–16 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Tajima T, Ibe M, Matsushita T, et al. A variety of skin responses to ultraviolet irradiation in patients with atopic dermatitis. J Dermatol Sci 1998;17:101–7 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (226.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Clinical implications of vitamin D deficiency
Beata matyjaszek-matuszek, monika lenart-lipińska, ewa woźniakowska.
- Author information
- Article notes
- Copyright and License information
Corresponding author: Beata Matyjaszek-Matuszek , MD, PhD, Department of Endocrinology, Medical University of Lublin, 8 Jaczewskiego St., 20-954 Lublin, Poland. phone: +48 81 724 46 68, fax: + 48 81 724 46 69, mobile: +48 604 302 289. e-mail: [email protected]
Corresponding author.
Received 2015 May 18; Revised 2015 May 19; Accepted 2015 May 19; Issue date 2015 Jun.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License, permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Vitamin D deficiency is a common medical problem worldwide and its prevalence rises along with latitude, obesity, sedentary lifestyle, limited sunlight exposure and aging. A great body of evidence has shown that patients with vitamin D deficiency have increased cardiovascular risks and total mortality. Conversely, the presence of comorbidities progressive with age such as abdominal obesity, insulin resistance, type 2 diabetes and hypertension places the patients at an increased risk of vitamin D deficiency. The multidirectional effect of vitamin D deficiency is present in different phases of the aging process. Based on the literature review, the risk factors for vitamin D insufficiency most often found in post-menopausal women include limited sun exposure and time spent outdoors, inadequate dietary vitamin D intake, winter season and increased age. Vitamin D supplementation in this group might offer prevention of falls and fractures and may be beneficial for cardiovascular health, what may be especially important in osteoporotic and elderly populations. Prevention and treatment processes involve education regarding sunlight exposure and pharmacological cholecalciferol supplementation according to the recommendations for Central Europe. This manuscript reviews the role of vitamin D and its deficiency and considers their clinical implications, with particular regard to peri- and postmenopausal women.
Keywords: vitamin D, deficiency, perimenopause, menopause
Introduction
In recent years, vitamin D (VD), a group of steroid compounds, has become of great interest due to many studies which have revealed its role far beyond bone metabolism. Through decades VD was considered a vitamin but nowadays it has emerged as an active hormone exerting its action as a transcription factor regulating the expression of numerous genes [ 1 ]. The presence of specific VD receptors (VDR) outside the skeletal system, enterocytes and renal tubular cells was confirmed in many cell types including immune cells, neurons, pancreatic cells, myocytes, cardiomyocytes, endothelium cells, which stress pleiotropic activity of VD. There is a great body of evidence confirming that apart from its well-known function in calcium-phosphate homeostasis, VD also exerts many non-calcemic actions in various tissues and systems. Vitamin D deficiency has been linked with significant complications such as cardiovascular events, obesity, metabolic syndrome, type 2 diabetes, various types of cancer, immune disorders, increased mortality and adverse pregnancy outcomes [ 2 – 4 ].
Currently, VD deficiency is considered a public health problem worldwide and its prevalence rises along with latitude, aging, sedentary lifestyle and limited sunlight exposure due to staying indoors or using sunscreen products. Furthermore, an increasing epidemic of obesity, which results in sequestration of VD in adipose tissue, also contributes to an increased risk of VD deficiency [ 2 , 5 ].
This manuscript reviews clinical implications of VD deficiency, its prevention and treatment, with particular regard to peri- and postmenopausal women.
Vitamin D metabolism
There are three ways to maintain adequate VD status: sunshine exposure, dietary intake and pharmaceutical supplementation. The major source of VD is its skin synthesis under the influence of solar ultraviolet B (UV-B) radiation which accounts for 80-90% of VD in humans [ 1 ]. In comparison to skin synthesis, the dietary supply of VD is minor and amounts only to 10-20% of total VD, however, it can become a significant source of VD if enriched with supplementation [ 1 ]. There are two types of physiologically important vitamins D: cholecalciferol (VD 3 ) mainly synthesized in the skin from 7-dehydrocholesterol upon exposure to UV-B and ergocalciferol (VD 2 ) obtained from diet. In the liver, VD is metabolized to 25-hydroxyvitamin D-25(OH)D by VD 25-hydroxylase (25-OHase) and further circulates to the kidneys where it undergoes a second hydroxylation by 25-hydroxyvitamin D-1αhydroxylase (1αOHase) to the biologically active form of VD – 1,25(OH) 2 D (calcitriol). The hydroxylation of VD in a position 1α is the key regulatory step in the bioactivation of VD and enzyme action in the kidneys is tightly controlled by numerous factors including serum parathormone (PTH), calcium, phosphate and various hormones such as estrogens, androgens, growth hormone, prolactin, thyroxin, cortisol and insulin [ 1 ]. The best indicator of the overall VD status is serum 25(OH)D concentration due to its long half-life (1-3 months) and metabolism which reflects total VD from dietary intake and sunlight exposure [ 6 ]. Although 1,25(OH) 2 D is more potent than 25(OH)D and has greater affinity to VDR it should never be used to determine VD status. This compound is present in circulation in a much lower concentration than 25(OH)D and has very short half-life (< 4 hours). Furthermore, the 1,25(OH) 2 D level may remain within the reference range or may even be elevated in the course of VD deficiency or secondary hyperparathyroidism [ 7 ]. The active form of VD combines with VDR and it has been established that VDRs are expressed in numerous tissues and cells. Accumulating evidence has shown that approximately 3% of the human genome may be regulated by VD, which indicates that VD deficiency is associated with significant clinical consequences [ 8 ].
The main causes of VD deficiency are limited skin synthesis due to inadequate sunlight exposure caused by sunscreen products overuse or limited outdoor activity as well as a low dietary intake of VD rich foods and many other factors such as aging, pigmented skin, smoking, obesity, air pollution, malabsorption and reduced synthesis due to liver or kidney diseases [ 7 ].
Diagnostic criteria of vitamin D deficiency
Serum 25(OH)D concentration is the best parameter to assess the overall VD status [ 9 ]. Current International Osteoporosis Foundation Guidelines recommended a target level of 30 ng/ml, which is associated with maximal suppression of PTH [ 9 ]. The diagnostic criteria of VD status are presented in Table I . There are several commercial assays available for the assessment of 25(OH)D which produce reliable results, but have notable bias in comparison with the reference HPLC method. It is worth knowing that the variation between laboratories may be as high as 30%. Liquid chromatography tandem mass spectroscopy (LC-MS) is considered the gold standard for the evaluation of VD status, due to commercially available methods such as RIA, ELISA, chemiluminescence assay may not measure all circulating forms of VD [ 11 , 12 ].
Diagnostic criteria of vitamin D status [ 10 ]
Vitamin D and obesity
The link between obesity and VD deficiency has been observed for years but determining the cause and effect has been difficult. Vimaleswaran et al . suggested that a higher BMI leads to a lower VD status whereas the effects of low VD status on BMI are likely to be marginal. In other words, these findings provide evidence for obesity as the causal factor for the development of VD deficiency but there is no proof that VD deficiency serves as the causal factor for the development of obesity [ 13 ]. Nonetheless, experimental studies have demonstrated that 1,25(OH) 2 D 3 plays an active role in adipose tissue by modulating inflammation, adipogenesis and adipocyte secretion as the key component of metabolic disorders e.g. in the metabolic syndrome [ 14 ]. A large study of the genetics underpinning both conditions finds that obesity may decrease VD levels but a predisposition to VD deficiency does not in fact lead to obesity. The findings also suggest that increasing VD levels will not reverse obesity. The fundamental mechanism that would explain why obesity suppresses VD is still discussed. Since VD is fat soluble, some scientists had assumed that it was sequestered in fatty tissues. If this was the case, less VD would reach the bloodstream. Nevertheless, while the vitamin is indeed stored in the adipose tissue, there is no evidence for sequestration of supplemental or endogenous cholecalciferol. Dilution of ingested or cutaneously synthesized VD in the large adipose tissue of obese patients fully explains their typically low VD status [ 15 ]. Therefore, the patients with BMI over 30 may require higher or more frequent doses of VD [ 16 ]. Nonetheless, Mason et al . found that vitamin D 3 supplementation during weight loss did not translate into higher body mass reduction or associated factors as compared with placebo, however, women who became replete experienced greater improvements [ 17 ].
Vitamin D and diabetes risk
Previous studies have yielded contradictory findings on the relationship between low VD and impaired glucose homeostasis. However, calcium is necessary to secrete insulin, which indirectly suggests that VD may in fact contribute to maintaining insulin secretion. Among the disorders linking VD deficiency to hyperglycemia we can find type 1 (T1DM), type 2 diabetes mellitus (T2DM) and metabolic syndrome. Type 1 diabetes mellitus and T2DM patients have a higher incidence of VD deficiency in comparison to the healthy population. The insulin-producing β-cells as well as numerous cell types of the immune system express VDR and vitamin D-binding protein and some allelic variations in genes involved in VD metabolism. What is more, its receptors are linked with glucose intolerance, insulin secretion and sensitivity as well as inflammation [ 18 ]. In the case of T1DM, VD supplementation in pre-diabetic individuals could help prevent or at least reduce the onset of autoimmune processes possibly by regulating thymic selection of the T-cell repertoire, decreasing the numbers of auto-reactive T cells and inducing Treg cells. Although immune modulation is widely discussed in the treatment of T1DM, it is also relevant in T2DM [ 19 ]. What is more, pharmacologic doses of 1,25-dihydroxyvitamin D (1,25(OH) (2) D) prevent insulitis and T1DM in non-obese diabetic mice and other models of T1DM. The possible reason behind this process may be immune modulation as well as direct effects on β-cell function [ 19 , 20 ]. Normal VD levels in the mother, and consequently in the fetus, decrease the risk of T1DM development in the child. The risk rises when maternal VD deficiency occurs in the second trimester as at this time i.e. around the 12 th week pancreatic β-cells are formed; while insulin secretion begins in the 20 th week of gestation. For that reason the mother should begin VD supplementation in the second trimester of gestation at the latest [ 21 ]. So far VD insufficiency has been known to serve as a T1DM risk factor, however, a growing body of evidence has pointed to its role in T2DM development. Vitamin D ameliorates the harmful biochemical impact of T2DM, possibly by increasing insulin secretion and sensitivity, improving the β-cell function, and decreasing the number of pro-inflammatory cytokines and insulin resistance [ 22 ]. Teegarden et al . confirmed that VD – or its active metabolite 1,25-dihydroxyvitamin D (1,25(OH) 2 D) – improves insulin sensitivity even in patients with glucose metabolism parameters with normal ranges. The proposed mechanisms possibly underlying this effect include potential relationships with improvements in lean mass, regulation of insulin release, altered insulin receptor expression and specific effects on insulin action [ 23 ]. Kramer et al . recognized VD deficiency and insufficiency with increased PTH is an independent predictor of such disorders as β-cell dysfunction, insulin resistance, and glycemia. The authors highlighted the need to consider the PTH/25-OH-D axis when studying the impact of VD status on glucose homeostasis [ 24 ]. Cho et al . showed VD 25(OH)D < 20 ng/ml deficiency in 85% of pregnant women with GDM and only in one out of every four pregnant women 25(OH)D deficiency in the first trimester was an independent risk factor for GDM and insulin resistance in the second trimester [ 25 , 26 ]. Vitamin D deficiency is linked not only with the risk of GDM but also with the prevalence of eclampsia, anemia or infections during gestation. It also affects the term of delivery and the birth weight [ 27 ]. Vitamin D supplementation improves insulin sensitivity and glucose tolerance; however, it has not been proven that it helps to prevent GMD [ 28 ].
Vitamin D and cardiovascular disease
Accumulating data suggest that VD may play a vital role for cardiovascular disease (CVD). The first evidence of the possible link between solar UV radiation and cardiovascular mortality showing an inverse relationship between these phenomena was reported in 1981 [ 29 ]. The expression of VDR and enzymes for vitamin D metabolism has been identified in the vascular system as well as in the heart. It has been reported that VDR knock-out mice suffer from cardiovascular disease and even selective VDR deletion in cardiomyocytes causes myocardial hypertrophy and exacerbates endothelial dysfunction [ 30 , 31 ]. Many observational studies confirmed the association of low VD status with a higher incidence of cardiovascular events and mortality. Zitterman et al . indicated a nonlinear decline in overall mortality as 25(OH)D concentrations increase [ 32 ]. Furthermore, low 25(OH)D concentrations have been reported as an independent risk factor for cardiovascular events, in particular for sudden cardiac deaths [ 33 ]. Vitamin D deficiency has been believed to be involved in insulin resistance, type 2 diabetes, and an adverse lipid profile [ 34 ].
Bjelakovic et al . in their meta-analysis evaluated the benefits and harms of vitamin D supplementation used in primary and secondary prophylaxis of mortality. They concluded that VD seemed to decrease mortality in elderly people [ 35 ]. However, some data have provided contradictory results. Ho et al . did not confirm that low VD is a significant risk factor for the presence of either calcific atherosclerosis or obstructive coronary artery stenosis [ 36 ]. Available evidence on the effects of VD on CVD has also been inconclusive. Gepner et al . failed to confirm the improvements in central blood pressure parameters or arterial stiffness in healthy postmenopausal women during VD supplementation [ 37 ]. Other researchers also reported no effect of VD supplementation on the cardiovascular risk factors [ 38 , 39 ].
Although, there is a great body of evidence that VD status may influence the risk of cardiovascular disease, final confirmation of a causal relationship between VD and the cardiovascular risk is still lacking. It is necessary to perform large randomized controlled supplementation trials with VD in specific risk groups to clarify the role of VD in cardiovascular events. Currently available RCTs on this topic are frequently limited by the additional supplementation of calcium which may increase the risk of CVD events. At present, the data are not sufficient for general recommendations to supplement VD in order to prevent and treat CVD [ 39 ].
Vitamin D in the carcinogenesis
The non-classical effects of VD, namely anti-proliferation, pro-differentiation, immune function modulation, and anti-inflammation, have been widely debated for the past decade. In particular, a lot of attention has been paid to the potential of VD analogs alone or in combination with other anticancer agents in the treatment of a variety of cancers. The relationship between VD status and the higher incidence of many types of cancer has suggested that VD may play a role in the etiology of these forms of cancer. Based on the present findings, 1α,25(OH) 2 D may have functions beyond its well-known action on calcium homeostasis and bone mineralization. The results of many studies have corroborated the fact that 1α,25(OH) 2 D exhibits anti-proliferative, pro-differentiating, anti-inflammatory, and pro-apoptotic functions in a tissue- and cell-specific manner. What is more, it has been shown to have a growth inhibitory effect on prostate, colon, breast, lung, liver and pancreatic cancer cells which express VDR [ 40 ]. The link between VD and breast mammographic density is a controversial problem. Heo et al . analyzed the correlation between 25-hydroxyvitamin D (25(OH)D) level and mammographic density in healthy pre- and postmenopausal women. The results of this study showed no significant associations between serum 25(OH)D and breast density [ 41 ]. Green et al . found no evidence of a correlation between plasma levels VD and mammographic density after menopause but their study suggested that high mammographic density and low plasma 25(OH)D level had four times greater risk of breast cancer than low mammographic density and high serum VD [ 42 ]. In the Women's Health Initiative Calcium and Vitamin D trial the authors observed no effect of VD and calcium supplementation on mammographic density after one year follow-up [ 43 ]. Epidemiological studies have suggested that low VD levels are associated with an increased risk of breast cancer. Vitamin D receptor is a crucial mediator for the cellular effects of VD. A molecular-based epidemiological study has identified several VDR genes and the overall data showed that the FokI and BsmI gene polymorphisms significantly correlate with an increased risk of ovarian and breast cancers. Mun et al . claimed that VDR polymorphisms may be potential risk factors for ovarian and breast cancers. The researchers implicate that in future studies VDR genetic variation should be integrated also with a pre-diagnostic indicator of VD status [ 44 ]. Both population and laboratory studies conducted on Polish female patients suffering from breast cancer have corroborated the important role of VD in the prevention of this disorder. An appropriate dietary intake and VD supplementation is a simple, safe and cost-effective method of reducing the risk of breast cancer development and should be recommended. The vital role of this vitamin has also been found in carcinogenesis of the ovarian cancer [ 45 ].
Vitamin D and menopausal period
The decrease in estrogens function observed during menopause, results in increased bone metabolism, a decrease in bone mineral density and the related elevated fracture risk. Furthermore, weight gain, decreased lean body mass and increased visceral fat tissue affect most women after menopause what places this group of patients at an increased risk of metabolic and cardiovascular disease. It has been well established that obesity is associated with VD deficiency [ 46 ]. This observation gives rise to the question whether VD deficiency is caused by obesity or obesity is a consequence of low VD levels. The data from the research suggest that rather obesity leads to VD deficiency, while the opposite connection seems not to be significant [ 47 ]. It has been reported that lower skin VD synthesis is observed with aging and even a similar exposure to solar radiation in elderly patients produces up to 75% less VD in comparison to young adults [ 48 ]. According to Gaugris et al ., the prevalence of low VD levels appears to be high in post-menopausal women, especially in those with osteoporosis and history of fracture [ 49 ]. Furthermore, PTH levels are higher in the elderly than in younger people at similar serum 25(OH)D levels, which may adversely affect the bone metabolism [ 50 ]. Additionally, the decline of estrogens after menopause decreases the activity of 1α-OHase, what results in lower synthesis of the active VD form. These results suggest that VD supplementation, even in higher doses, may be necessary in postmenopausal women to overcome high parathyroid activity, also probably exacerbated by a decreased renal function. Vitamin D supplementation seems to be the most appropriate treatment option for the population of postmenopausal patients and has been suggested by many experts as a safe and cost-effective procedure. However, the role of VD supplementation in the prevention and treatment of comorbidities associated with aging and menopausal consequences has not been completely established. Płudowski et al . offer elaborated consensus on supplementation guidance and population strategies for VD in Central Europe and recommend that the elderly (65 years and above) should be supplemented with 800-2,000 IU/day (20.0-50.0 µg/day) throughout the whole year, because of the reduced efficacy of VD skin synthesis. In obese elderly patients, supplementation of 1,600-4,000 IU/day (40-100 µg/day), depending on severity of obesity, is recommended throughout the whole year [ 10 ].
Menopausal symptoms and vaginal atrophy
Menopause is characterized by falling levels of estrogen and progesterone, which can lead to the development of symptoms including hot flushes, night sweats, and vaginal dryness. As it has already been discussed, the decline in estrogens also results in the increased bone turnover, decrease in bone mineral density and elevated risk of fracture. The quality of life may be impaired by musculoskeletal discomfort, frequent mood disturbances and an increased risk of metabolic and cardiovascular diseases. Capatina et al . studied VD status and biological correlations in postmenopausal women and demonstrated a widely prevalent insufficiency as only 8.1% had sufficient VD levels [ 51 ]. The Women's Health Initiative trial found borderline significant associations between 25(OH)D levels and menopausal symptoms such as sleep disturbance, emotional well-being, and energy/fatigue [ 52 ]. Most of the postmenopausal women suffer from vulvovaginal atrophy, dryness and irritation associated with estrogen deficiency. Therefore, safe treatment is desirable to enhance vaginal lubrication. Vitamin D is known to be involved in the regulation of growth and differentiation of body cells, especially squamous epithelium, present in the vagina. Therefore, this vitamin could be effective in proliferation and repair of epithelial vaginal tissue. Yildirim et al . have demonstrated that VD supplementation resulted in squamous maturation of the vaginal epithelium. These findings suggest that there must be an intracellular receptor in vaginal epithelium. No evidence has been found regarding the side effects of VD [ 53 ]. Rad et al . demonstrated that VD vaginal suppositories improve the maturation index and decrease the pH and dryness in women with vaginal atrophy due to menopause. The authors suggested that vaginal suppositories may be used as lubricants and moisturizers and they can be a new strategy of vaginal atrophy treatment in many women with contraindication to estrogen therapy which include among others the history of breast cancer, stroke and venous thromboembolism [ 54 ]. However, other studies, like the one conducted by Shirazi et al ., found no association between menopausal status and serum VD levels [ 55 ].
Urogenital symptoms
Only few studies in the literature have assessed the relationship between VD status and pelvic floor disorders. Parker et al . showed that insufficient VD is linked to a greater impact of urinary incontinence on women's quality of life [ 56 ]. Their longitudinal cohort study suggested that low intake levels of certain micronutrients may be associated with an increased risk of overactive bladder (OAB), the most significant association being with VD. Dallosso et al . reported that a higher VD intake significantly correlated with a reduced risk of OAB in women aged 40 and older [ 57 ]. The authors assumed that VD plays an important role in skeletal muscle efficiency and potentially in the detrusor muscle and urothelial function. Thus, they explained their observation that VD deficiency has more effects on urinary incontinence in comparison to VD sufficient women [ 56 , 57 ]. Gau et al . reported only two cases of urgency urinary incontinence with high dose VD supplementation [ 58 ]. Vitamin D receptor has been identified in the urothelium and the smooth muscle of the detrusor wall [ 59 ]. Vitamin D insufficiency may also affect the detrusor wall contributing to symptoms of OAB and urgency urinary incontinence (UUI). Badalian et al . demonstrated the results of the National Health and Nutrition Examination Survey and suggested that higher VD levels are associated with a decreased risk of pelvic floor disorders in women. The authors revealed that the risk of urinary incontinence was 45% lower in people with VD levels of 30 ng/ml or higher than in those with inadequate levels [ 60 ].
Conclusions
The latest scientific findings have stressed the pleiotropic action of VD and defined the prominent role of this vitamin in the pathomechanisms of numerous disorders. The multidirectional effect of VD deficiency is present in different phases of the aging process. Its negative metabolic consequences as well as higher prevalence of cardiovascular events and increase in overall mortality points to the need to treat the deficiency or – even better – to prevent it. Randomized clinical and placebo controlled trials have found that 25(OH)D 30-80 ng/ml VD has positive effects and helps induce beneficial clinical changes. Prevention and treatment processes involve education regarding sunlight exposure and pharmacological cholecalciferol supplementation according to the recommendations for Central Europe.
Authors report no conflicts of interest.
- 1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Rosner CJ. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int J Womens Health. 2013;5:523–531. doi: 10.2147/IJWH.S51403. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Kouda K, Nakamura H, Fujita Y, et al. Vitamin D status and body fat measured by dual-energy X-ray absorptiometry in a general population of Japanese children. Nutrition. 2013;29:1204–1208. doi: 10.1016/j.nut.2013.03.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Zitterman A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Płudowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2009;60:68–75. doi: 10.5603/ep.2013.0012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98:1283–1304. doi: 10.1210/jc.2013-1195. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Mutt SJ, Hyppönen E, Saarnio J, et al. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;24:228–229. doi: 10.3389/fphys.2014.00228. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Pathak K, Soares MJ, Calton EK, et al. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014;15:528–537. doi: 10.1111/obr.12162. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Mason C, Xiao L, Imayama I, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. Am J Clin Nutr. 2014;99:1015–1025. doi: 10.3945/ajcn.113.073734. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Takiishi T, Gysemans C, Bouillon R, et al. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–446. doi: 10.1016/j.ecl.2010.02.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Takiishi T, Gysemans C, Bouillon R, et al. Vitamin D and diabetes. Rheum Dis Clin North Am. 2012;38:179–206. doi: 10.1016/j.rdc.2012.03.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94–111. [ PubMed ] [ Google Scholar ]
- 22. Sadek KM, Shaheen H. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharm Biol. 2014;52:591–596. doi: 10.3109/13880209.2013.854812. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Teegarden D, Donkin SS. Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Kramer CK, Swaminathan B, Hanley AJ, et al. Prospective associations of vitamin D status with β-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. 2014;63:3868–3879. doi: 10.2337/db14-0489. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Cho GJ, Hong SC, Oh MJ, et al. Vitamin D deficiency in gestational diabetes mellitus and the role of the placenta. Am J Obstet Gynecol. 2013;209:560.e1–8. doi: 10.1016/j.ajog.2013.08.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Lacroix M, Battista MC, Doyon M, et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014;5:609–616. doi: 10.1007/s00592-014-0564-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Aghajafari F, Nagulesapillai T, Ronksley PE, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Burris HH, Camargo CA. Vitamin D and gestational diabetes mellitus. Curr Diab Rep. 2014;14:451. doi: 10.1007/s11892-013-0451-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol. 1981;10:337–341. doi: 10.1093/ije/10.4.337. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Ni W, Watts SW, Ng M, et al. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64:1290–1298. doi: 10.1161/HYPERTENSIONAHA.114.03971. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Zittermann A, Iodice S, Pilz S, et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Drechsler C, Pilz S, Obermayer-Pietsch B, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–2261. doi: 10.1093/eurheartj/ehq246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Skaaby T, Husemoen LL, Pisinger C, et al. Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology. 2012;123:62–70. doi: 10.1159/000341277. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;10:CD007470. doi: 10.1002/14651858.CD007470.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Ho JS, Cannaday JJ, Barlow CE. Low 25-OH vitamin D levels are not associated with coronary artery calcium or obstructive stenoses. Coron Artery Dis. 2015 doi: 10.1097/MCA.0000000000000261. [Epub ahead of print] [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Gepner AD, Haller IV, Krueger DC, et al. A randomized controlled trial of the effects of vitamin D supplementation on arterial stiffness and aortic blood pressure in Native American women. Atherosclerosis. 2015;240:526–528. doi: 10.1016/j.atherosclerosis.2015.04.795. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Beveridge LA, Struthers AD, Khan F, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015;175:745–754. doi: 10.1001/jamainternmed.2015.0237. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer. 2013;49:1422–1436. doi: 10.1016/j.ejca.2012.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Heo DS, Lee JG, Hwang HR, et al. The association between 25-hydroxyvitamin D and mammographic density in healthy pre- and postmenopausal women regardless of the menstrual cycle phase: a cross-sectional study. Nutr Cancer. 2014;66:97–103. doi: 10.1080/01635581.2014.851715. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Green AK, Hankinson SE, Bertone-Johnson ER, et al. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127:667–674. doi: 10.1002/ijc.25075. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Bertone-Johnson ER, Chlebowski RT, Manson JE, et al. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010;17:1152–1160. doi: 10.1097/gme.0b013e3181e102d9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Mun MJ, Kim TH, Hwang JY, et al. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas. 2015;81:256–265. doi: 10.1016/j.maturitas.2015.03.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Walentowicz-Sadłecka M, Sadłecki P, Walentowicz P, et al. The role of vitamin D in the carcinogenesis of breast and ovarian cancer. Ginekol Pol. 2013;84:305–308. doi: 10.17772/gp/1581. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc. 2014;31:1–10. doi: 10.1017/S0029665114001578. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Hagenau T, Vest R, Gissel TN, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20:133–140. doi: 10.1007/s00198-008-0626-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Gaugris S, Heaney RP, Boonen S, et al. Vitamin D inadequacy among post-menopausal women: a systematic review. QJM. 2005;98:667–676. doi: 10.1093/qjmed/hci096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–191. doi: 10.1210/jc.2002-021064. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Capatina C, Carsote M, Caragheorgheopol A, et al. Vitamin D deficiency in postmenopausal women – biological correlates. Maedica (Buchar) 2014;9:316–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. LeBlanc ES, Desai M, Perrin N, et al. Vitamin D levels and menopause-related symptoms. Menopause. 2014;21:1197–1203. doi: 10.1097/GME.0000000000000238. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Yildirim B, Kaleli B, Düzcan E, et al. The effects of postmenopausal Vitamin D treatment on vaginalatrophy. Maturitas. 2004;10:334–337. doi: 10.1016/j.maturitas.2004.02.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Rad P, Tadayon M, Abbaspour M, et al. The effect of vitamin D on vaginal atrophy in postmenopausal women. Iran J Nurs Midwifery Res. 2015;20:211–215. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Shirazi L, Almquist M, Malm J, et al. Determinants of serum levels of vitamin D: a study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC Womens Health. 2013;15:13–33. doi: 10.1186/1472-6874-13-33. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Parker-Autry CY, Markland AD, Ballard AC, et al. Vitamin D status in women with pelvic floor disorder symptoms. Int Urogynecol J. 2012;23:1699–1705. doi: 10.1007/s00192-012-1700-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Dallosso HM, McGrother CW, Matthes RU, et al. Nutrient composition of the diet and the development of overactive bladder: a longitudinal study in women. Neurourol Urodynam. 2004;23:204–210. doi: 10.1002/nau.20028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Gau JT. Urinary incontinence resolved after adequate vitamin D supplementation: a report of two cases. JAGS. 2010;58:2438–2439. doi: 10.1111/j.1532-5415.2010.03179.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Crescioli C, Morelli A, Adorini L, et al. Human bladder as a novel target for vitamin D receptor ligands. J Clin Endocrinol Metab. 2005;90:962–972. doi: 10.1210/jc.2004-1496. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Badalian SS, Rosenbaum PF. Vitamin D and pelvic floor disorders in women: results from the National Health and Nutrition Examination Survey. Obstet Gynecol. 2010;115:795–803. doi: 10.1097/AOG.0b013e3181d34806. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (284.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

COMMENTS
This was a non-classical case of vitamin D deficiency of a 26-year-old woman who presented with chronic anosmia and recurrent nasal polyps. She was diagnosed as having a case of vitamin D deficiency and responded well to vitamin D replacement therapy.
The re-emergence of vitamin D deficiency in westernized societies is thought to be multifactorial secondary to poor dietary intake, popularization of breastfeeding, and diminished exposure to sunlight.
Research has shown that vitamin D deficiency has serious clinical consequences, including cardiovascular diseases, cancer, and diabetes. It can also lead to loss of bone density, which contributes to osteoporosis and fractures . In children, severe vitamin D deficiency can lead to rickets.
In the present report, we describe a case of osteomalacia caused by vitamin D deficiency in an adult patient who strictly complied with dietary restrictions and avoidance of sun exposure to manage her worsening atopic dermatitis.
Vitamin D deficiency is a common medical problem worldwide and its prevalence rises along with latitude, obesity, sedentary lifestyle, limited sunlight exposure and aging. A great body of evidence has shown that patients with vitamin D deficiency have increased cardiovascular risks and total mortality.
We herein report the case of a 41-year-old Japanese female office worker who developed symptomatic hypocalcemia with severe vitamin D deficiency following treatment for Graves’ disease with methimazole.