An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


The End of Behavioral Genetics?
- Author information
- Copyright and License information
Correspondence: Matt McGue, Department of Psychology, University of Minnesota, 75 East River Rd., Minneapolis, MN 55455, (612)-625-8305 (Voice); (612)-626-2079 (FAX), [email protected]
Although genetic models were in the ascendance within psychology during the early 20 th century, the association of early behavioral genetic research with the eugenics movement served to discredit the field in the eyes of many. Twin and adoption studies throughout the latter half of the 20 th century helped to reestablish the importance of behavioral genetic models and set the stage for the current focus of the field on developing and testing models of gene-environment interplay. Research findings on developmental behavioral genetic research, gene-environment interaction, and the use of behavioral genetic models to test causal hypotheses are used to highlight the contributions of contemporary behavioral genetic research to psychological research. It is argued that future efforts to investigate models of gene-environment interplay will depend heavily of the field’s ability to identify the specific genetic variants that contribute to individual differences in behavior. The anticipated yield from genome-wide association studies gives much reason to be optimistic about the future vitality of behavior genetics.
The end of behavioral genetics? Recently the developmental psychologist, Richard Lerner, published a commentary entitled, “Another nine-inch nail for behavioral geneticists!” ( Lerner, 2006 ). Lerner began his commentary with the lament, “Why do we have to keep reinterring behavior genetics” (p. 337), but concluded confident in the belief that a critical analysis of IQ adoption studies ( Richardson & Norgate, 2006 ) had finally help put the field irretrievably where he felt it belonged, presumably six feet under. Singing the death knell of behavior genetics has been a popular pastime for critics of the field over the past 100 years, but critiques like that by Richardson and Norgate (2006) have done little to dampen interest in behavioral genetics. It is not that behavioral genetics is above criticism; quite the contrary there is much to criticize about the field. Rather, the reason Lerner and his fellow critics are engaged in the Sisyphean task of “reinterring” behavioral genetics is that they have failed to recognize the enormous impact the field has had on the way psychologists think about individual differences in behavior; they have failed to understand the essential nature of the behavioral genetic research paradigm.
The purpose of this special issue of Acta Psychologica Sinica is, of course, to recognize the vitality of contemporary behavioral genetic research, not to mourn its demise. Nonetheless, even as we highlight research progress in the field, it can be helpful to consider how others have reacted to behavioral genetic findings, even the field’s most enthusiastic critics. Behavioral genetics has often been caught up in controversy, sometimes unfairly, and an assessment of its current impact on psychology can benefit from both a discussion of the field’s historical roots, with which I will begin, and speculation over its possible future, where I will end.
The specific charge for this special issue is to highlight contemporary behavioral genetic research. In a single article it is no longer possible to comprehensively survey the full range of behavioral genetic research. As an alternative, I have elected to highlight new developments in behavioral genetics by describing how selected findings from the University of Minnesota, and especially from the Minnesota Center for Twin and Family Research (MCTFR), fit in with larger developments in the field. The MCTFR was begun in 1988 by David Lykken, Auke Tellegen, William Iacono and myself when we initiated the Minnesota Twin Family Study (MTFS), a longitudinal study of approximately 1400 families, each consisting of a pair of adolescent like-sex twins and their parents ( Iacono, McGue, & Krueger, 2007 ). We have continued to follow these families every 3–4 years as the twins have passed through mid- and late-adolescence through their early to late 20s ( McGue & Iacono, 2008 ). In 1998, we initiated the Sibling Interaction and Behavior Study (SIBS), a parallel longitudinal study of 409 adoptive and 208 non-adoptive families, each consisting of a pair of adolescents and their rearing parents ( McGue et al., 2007 ). As in the MTFS, the adolescent participants in SIBS have been followed through adolescence into early adulthood. Together, MTFS and SIBS provide a powerful foundation for engaging in developmental behavioral genetic research and addressing the important research questions that define the field today.
BEHAVIORAL GENETICS: THE HISTORICAL CONTEXT
The early rise and early demise of behavioral genetics.
When the nascent field of psychology was taking form at the end of the 19 th century, it had a decidedly Darwinian orientation. In large measure this owed to the efforts of Francis Galton, Charles Darwin’s cousin. Galton was a polymath who made an extraordinary number of important contributions to science. He developed the fingerprint method still used today in forensics, started the field of biometry with his student Karl Pearson, and was one of the founders of the journal Nature ( Gillham, 2001 ). His cousin’s publication of the Origin of Species led Galton to dedicate his scientific career to the application of Darwinian principles to human behavior. His 1869 publication, Hereditary Genius ( Galton, 1869 ), is the first rigorous scientific investigation of the familial transmission of a human behavioral trait, in this case social achievement, and inaugurated the field of human behavioral genetics. Hereditary Genius and other empirical investigations led Galton to the conclusion that, “nature prevails enormously over nurture when the differences of nurture do not exceed what is commonly to be found among persons of the same rank in society and in the same country” ( Galton, 1876 ) (p. 404)
Galton believed inherited factors had a substantial influence on individual differences in behavior. As the quote indicates, however, he did not believe that nurture was unimportant, especially at the extremes of economic deprivation and privilege. Nonetheless, the ascendance of biological thinking in the early days of psychology led some to advocate a doctrine of genetic determinism. Henry Goddard, one of the leading figures in developmental psychology in the early 20 th century, argued that, “… grade of intelligence or mental level for each individual is determined by the kind of chromosomes that come together with the union of the germ cells … [and] is but little affected by any later influence …” (p.1) ( Goddard, 1920 ). For Goddard, and others like him, our fates were largely sealed at the moment of conception and little affected by families, schools and social institutions. Ironically, the belief in genetic determinism when combined with another of Galton’s intellectual contributions undermined his early efforts to place psychology on a behavioral genetic foundation. In 1883, Galton (1883) introduced the term eugenics (Greek for well [eu] born [genic]) to denote how knowledge of the principles of the inheritance of human behavior might be applied to pressing social problems: “ … if talented men were mated with talented women, of the same mental and physical characters as themselves, generation after generation, we might produce a highly-bred human race, with no more tendency to revert to meaner ancestral types than is shown by our long-established breeds of race-horses and fox-hounds.” ( Galton, 1865 ) (p. 318). Even if Galton’s original conceptualization of eugenics was relatively benign (i.e., emphasizing educational efforts or, at the most extreme, “positive” eugenic practices such as paying the ‘most talented’ to reproduce), it eventually devolved into repressive proscriptive efforts (i.e., forced sterilization and restrictive immigration laws). As most know, eugenics was ultimately seized upon by the Nazis in an attempt to provide a scientific rationale for their racial hygiene laws and the Holocaust.
Behavioral genetics was nearly completely discredited by its early association with the eugenics movement. Few intellectuals wanted to be associated with a scientific endeavour perceived to have contributed to the Nazi’s repressive policies, no matter how indirectly. The demise of biological accounts within psychology created a vacuum that was filled by a view of human nature, tabula rasa, that was arguably every bit as radical as that offered by the genetic determinists ( Pinker, 2002 ). Many will be familiar with the following boast from John B. Watson, the founder of radical behaviorism: “Give me a dozen healthy infants well-formed, and my own specified world to bring them up in and I’ll guarantee to take any one at random and train him to become any type of specialist I might select -- a doctor, lawyer, artist, merchant-chief, and, yes, even beggar-man and thief, regardless of his talents, penchants, tendencies, abilities, vocations, and race of his ancestor.” ( Watson, 1930 ) (p.82). Less familiar may be the next sentence in Watson’s quote, “I am going beyond my facts and I admit it, but so have the advocates of the contrary and they have been doing it for many thousands of years.”, which indicates that Watson knew he was being hyperbolic. Whether Watson could make good on his boast was largely irrelevant, however, as purely environmental accounts would dominate within psychology and psychiatry for much of the next 50 years. For example, schizophrenia was attributed to the machinations of mothers (i.e., the “schizophrenic mother”) ( Fromm-Reichmann, 1948 ), while personality was considered to be a reflection of the home you grew up in ( Mischel, 1981 ). By the mid-20 th century, the “Blank Slate” model of human nature had taken a strong hold on the field ( Harris, 1998 ).
Twin and Adoption Studies and the Reemergence of Behavioral Genetic Research
Even though it had lost most of its popularity by the middle of the 20 th century, behavioral genetics was not without its adherents. Initially in Western Europe and increasingly in the U.S., a small group of researchers undertook a set of investigations that would challenge and eventually undermine the Blank Slate model. Using traditional research methodologies such as adoption and twin studies, these researchers accumulated a set of research findings that in aggregate provided compelling evidence for the existence of genetic influences on individual differences in a wide range of behavioral outcomes.
Adoption Studies
The logic of the adoption study is relatively straightforward: If genetic factors are important, then individuals should to some degree resemble their biological parents regardless of whether they had been reared by those parents. This is precisely what behavioral geneticists have repeatedly found. For traits as diverse as intellectual ability ( Teasdale & Owen, 1984 ), schizophrenia ( Heston, 1966 ), mood disorders ( Mendlewicz & Rainer, 1977 ), alcoholism ( Cloninger, Bohman, & Sigvardsson, 1981 ), and criminal behavior ( Mednick, Gabrielli, & Hutchings, 1984 ) researchers have reported significant resemblance between biological parents and their reared-away offspring. At the same time, and somewhat unexpectedly, many but not all of these researchers also failed to find significant behavioral resemblance among adoptive relatives. For example, being reared by a parent with schizophrenia ( Wender, Rosenthal, Ketty, Schulsinger, & Welner, 1974 ); an affective disorder ( Mendlewicz & Rainer, 1977 ), or criminality ( Mednick et al., 1984 ) was not associated with increased offspring risk so long as they were not genetically related to the rearing parents. These early findings hinted at what now is a fairly well established behavioral genetic finding: While environmental effects on human behavior are pervasive and strong, they appear predominantly to contribute to differences rather than similarities among reared-together relatives ( Plomin & Daniels, 1987 ).
Reared-together Twin Studies
By far the most common behavioral genetic design is the study of reared-together twins. Twins are relatively common, in some countries accounting for as much as 2–4% of the population, and the existence of national registries in many countries ( Busjahn & Hur, 2006 ; Kaprio, 2006 ; Lichtenstein et al., 2006 ; Skytthe et al., 2006 ) and some U.S. states ( Lykken, Bouchard, McGue, & Tellegen, 1990 ; Rhea, Gross, Haberstick, & Corley, 2006 ) has made it relatively easy to undertake large-scale studies of twins. Although more recent twin registries are being established in many Asian countries as well ( Ando et al., 2006 ; He, Ge, Zheng, Huang, & Zeng, 2006 ; Hur, Shin, Jeong, & Han, 2006 ; Li et al., 2006 ; Pang et al., 2006 ). In a twin study, the inference of the existence of a genetic influence is based on the similarity of the two types of twins. That is, if genetic factors are important, then monozygotic (MZ) twins, who are genetically identical, should be more similar than (same-sex) dizygotic (DZ) twins, who like ordinary siblings share 50% of their segregating genes.
In multiple studies of behavioral traits as diverse as intellectual ability ( McGue, Bouchard, Iacono, & Lykken, 1993 ), personality ( Finkel & McGue, 1997 ), psychopathology ( I.I. Gottesman & Shields, 1972 ) and even social attitudes ( Martin et al., 1986 ) and divorce ( McGue & Lykken, 1992 ) behavioral geneticists have reported greater similarity among MZ than DZ twins. Figure 1 provides an example of the nature of these findings in terms of twin concordances (i.e., risk to the cotwins of affected individuals) for various types of behavioral disorders drawn from a review of behavioral genetic research ( McGue & Bouchard, 1997 ). As can be seen, even though concordances vary from one disorder to the next, they are always higher among MZ as compared to DZ twins, suggesting that genetic factors influence psychopathology risk. Alternatively, concordances among MZ twins are never perfect and indeed generally fall in the moderate range, .4 to .6. Since MZ twins are genetically identical, this lack of perfect concordance must be due to environmental factors, albeit those that contribute to differences rather than similarities among family members since in the case of Figure 1 all twins have been reared-together.
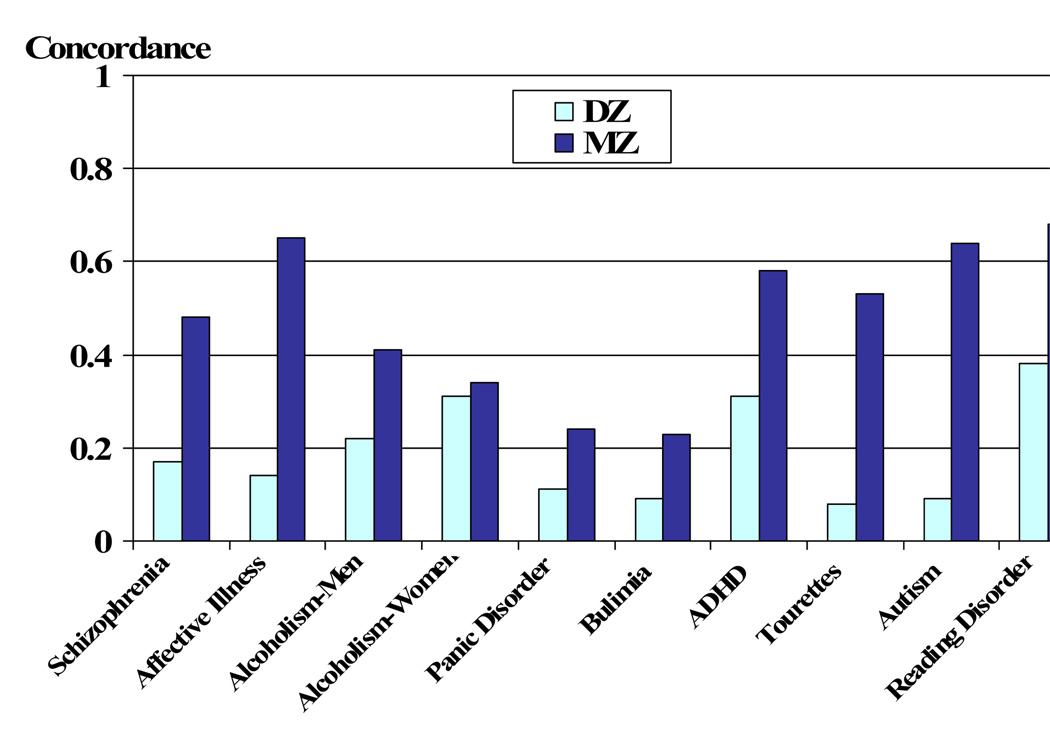
Risk to monozygotic (MZ) and dizygotic (DZ) cotwins of twins affected with various mental disorders. Adapted from McGue and Bouchard ( McGue & Bouchard, 1997 ).
Reared-Apart Twin Studies
Far less common than either of the two traditional methodologies used by behavioral geneticists is their intersection, the study of adopted twins. There have been approximately a half dozen systematic investigations of reared-apart twins published in the literature and findings from these studies provide constructive replication of findings from adoption and reared-apart twin studies. That is, there is significant psychological similarity between the two members of an MZ twin pair even when they have been reared in separate homes from infancy. This is perhaps made most evident by findings from the Minnesota Twin Study of Twins Reared Apart (MISTRA), which reported similarity for a range of psychological characteristics in a sample of more than 100 pairs of reared-apart twins ( T. J. J. Bouchard, Lykken, McGue, Segal, & Tellegen, 1990 ). Figure 2 summarizes findings from this study in terms of the correlation among reared-together and reared-apart twins averaged across multiple indicators clustered in four major domains of psychological functioning. Three general trends are evident. First, reared-apart MZ twin similarity ranges from moderate (for social attitudes) to strong (for IQ), implicating genetic influences, albeit of varying magnitude, across a range of psychological outcomes. Second, MZ twin similarity in each domain is never perfect, implicating the existence of environmental influences, which for social attitudes and interests account for the majority of phenotypic variance. Finally except for IQ, reared-together MZ twins are not much more similar than reared-apart MZ twins, suggesting that common rearing may not have a substantial effect on individual differences in the domains represented.
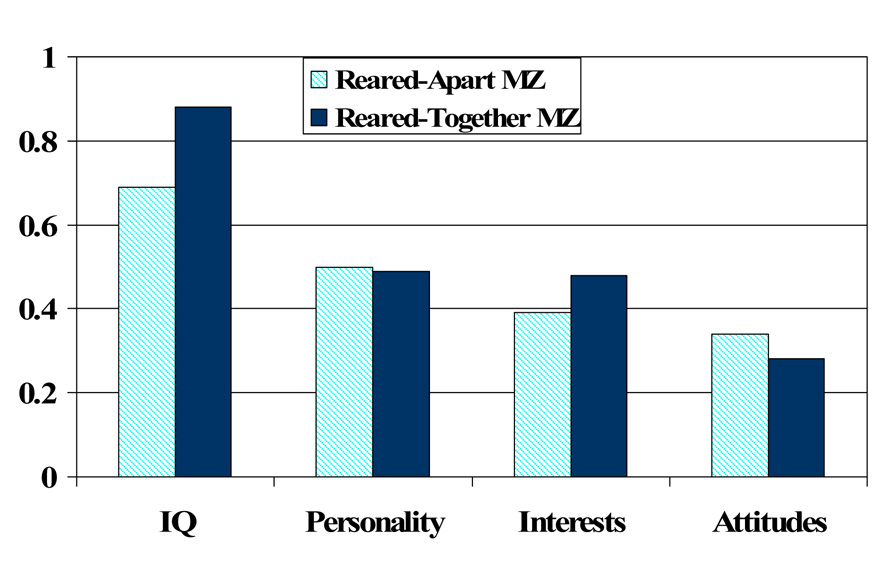
Average reared-apart and reared-together monozygotic (MZ) twin correlations in four domains of psychological functioning. Adapted from Bouchard et al. (1990)
Biometrical Methods of Analysis
Qualitative analyses of twin and adoption studies support the existence of both genetic and environmental influences on a broad range of behavioral outcomes. Quantitative analysis of twin and family data help to strengthen and refine these conclusions by estimating effect size, assessing statistical model fit, and identifying trends in the data that might not otherwise be apparent. Biometrical analysis involves fitting explicit models of genetic and environmental influence to twin and family data ( Neale & Cardon, 1992 ). The biometrical approach is based on the assumption that variance in a quantitative phenotype (e.g., IQ, a personality scale score, or a measure of speed of response) can be decomposed into its underlying determinants, with most research focusing on the following three major variance components: additive genetic factors, shared environmental factors (i.e., those environmental factors that are shared by reared together relatives and thus potentially contribute to their behavioral similarity), and non-shared environmental factors (i.e., those environmental factors that are not shared by reared-together relatives and thus potentially contribute to their behavioral differences). Because the biometrical approach is based on a set of simplifying assumptions (e.g., most biometrical analyses do not make explicit allowance for gene-environment interaction effects), results using this approach are at best approximations. Nonetheless, these approximations have proven to provide a useful initial description of the major contributors to individual differences in behavior.
Biometrical models can be fit to individual studies; they can also be fit to data aggregated across multiple studies. The study by Chipuer et al. ( Chipuer, Rovine, & Plomin, 1990 ) illustrates both the biometrical approach and the use of aggregated data. These researchers fit various biometrical models to the aggregate familial IQ correlations data published by Bouchard and McGue ( T. J. Bouchard & McGue, 1981 ). Qualitative analysis of the twin and family correlations implicated the existence of genetic influences on IQ (e.g., the pooled MZ correlations for IQ was .86 on 4672 pairs, while the comparable value for DZ twins was .60 on 5533 pairs). Biometrical analysis allowed these investigators to formalize and quantify impressions gained through informal qualitative analyses. Based on the fitted parameter estimates, these researchers concluded that approximately 51% of IQ variance was associated with genetic factors, 35% with shared environmental factors, and 14% with non-shared environmental factors. Subsequent studies have reported similar summary estimates for IQ ( Devlin, Daniels, & Roeder, 1997 ).
CONTEMPORARY BEHAVIORAL GENETIC RESEARCH
The focus on establishing the existence of heritable influences on behavior that dominated within the field throughout much of the twentieth century led to concerns that behavioral geneticists were seeking to resurrect the doctrine of genetic determination ( Feldman & Lewontin, 1975 ). Even if historically grounded, these critiques failed to recognize the need to reestablish the significance of genetic factors as a counterforce to Blank Slate approaches. Indeed, if anything, behavioral genetic research has done more to undermine than support deterministic arguments ( Bioethics, 2002 ). Establishing unequivocally the importance of genetic contributions to behavior in the 20 th century has allowed behavioral geneticists to move on to explore more important questions about the mechanisms by which genetic and environmental factors combine to jointly influence behavior in the 21 st ( Rutter & Silberg, 2002 ). A systematic review of contemporary behavioral genetic research is well beyond the scope of the present article. As an alternative, we illustrate the focus of contemporary behavioral genetic research on joint models of genetic and environmental effects by describing selected findings from the MCTFR as well as related findings from the larger field.
Behavioral Genetics and Development
One of the more provocative findings to emerge from the behavioral genetic literature is the observation that genetic influences on behavior appear to increase with age while shared environmental influences wane. Although this pattern was first observed with IQ ( McCartney, Harris, & Bernieri, 1990 ; McGue et al., 1993 ), the same pattern has been reported for a wide range of behavioral traits including political attitudes ( Eaves et al., 1997 ), antisocial behavior ( Lyons et al., 1995 ), parent-child relations ( McGue, Elkins, Walden, & Iacono, 2005 ), religiousness ( L. B. Koenig, McGue, & Iacono, 2008 ; L.B. Koenig, McGue, Krueger, & Bouchard, 2005 ), and peer group affiliation ( Kendler et al., 2007 ). In a recent meta-analysis, Bergen et al. ( Bergen, Gardner, & Kendler, 2007 ) showed that this pattern was most evident during the transition from adolescence to early adulthood, a developmental period also characterized by increasing individual control over the nature and range of experience.
Rather than interpreting increasing genetic influence as evidence of the increasing genetic determination of behavior, most behavioral geneticists interpret this pattern as implicating processes of gene-environment interplay. Anticipating developmental behavioral genetic findings from the last 15 years, Scarr and McCartney ( Scarr & McCartney, 1983 ) proposed a model of gene-environment correlation that predicts developmental changes in heritable effects. Behavioral geneticists distinguish three major processes by which genetic and environmental effects can come to be correlated. Passive gene-environment correlation arises because parents who transmit genes to their children also help to create the environments their children are reared in. For example, high-ability parents not only transmit to their children genes that support intellectual achievement, they are also likely to provide a rearing environment that is intellectual enriched. Evocative gene-environment correlation arises because the environments individuals experience are in part a function of the way parents, teachers, friends, and others react to their (genetically-influenced) behavior. For example, the social environments experienced by two children, the first overactive and oppositional, the second restrained and compliant, are likely to be markedly different in part because of differences in the ways the two children act. Finally, active gene-environment correlation refers to the process by which individuals actively construct their environments by selecting experiences that reinforce or complement their inherited abilities, dispositions and interests.
Scarr and McCartney (1983) hypothesized that as individuals grow older they gain increasing control over experiential choices, which consequently increasingly reflect their genetically-influenced propensities. These increases in active gene-environment correlational processes are accompanied by decreases in passive gene-environmental effects reflecting diminishing parental influences. The result is the classic pattern of developmental increases in heritability and decreases in shared environmental influences. Research at the MCTFR supports the importance of gene-environment correlational processes during adolescence and early adulthood. For example, we have found that individuals who engage in problem behavior early in adolescence are at a substantial increased risk of substance abuse and mental health problems in early adulthood ( McGue & Iacono, 2005 ) and that this association is due primarily to genetic effects common to early problem behavior and adult mental health problems ( McGue, Iacono, & Krueger, 2006 ). At the same time, our findings do not support genetic determination of mental health outcomes, as early problem behavior appears to affect adult risk in part because its expression increases the likelihood that the developing adolescent is exposed to deviant peers, has a conflicted relationship with his or her parents, and is disengaged from school ( M. A. Keyes, Iacono, & McGue, 2007 ).
Gene-Environment Interaction: A New Behavioral Genetic Paradigm
Gene-environment interaction (G×E) refers to non-additive combination of genetic and environmental effects on phenotype. Although long a focus of theoretical interest, the systematic investigation of G×E in human behavioral genetics has only recently been made feasible by the development of both biometric approaches, which provide overall tests for the existence of G×E ( Purcell, 2002 ), and measured genotype approaches, which test hypotheses about the interaction of specific functional genetic polymorphisms with specific environmental agents ( Moffitt, Caspi, & Rutter, 2005 ). The growing interest in G×E can be seen in Figure 3 , which charts entries to “gene environment interaction” in the Thomson Institute for Scientific Information (ISI) database over the past 20 years. In 1988 there was not a single entry to G×E in the ISI database; last year there were more than 400. G×E clearly represents a major paradigm shift among those investigating genetic contributions to human health and well being.
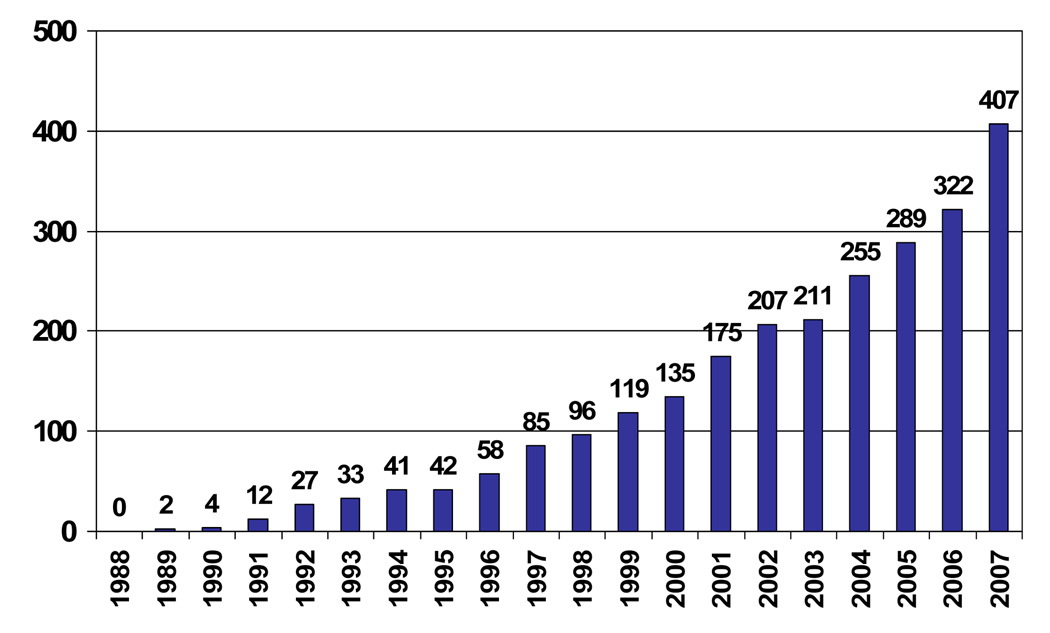
Number of entries to “Gene Environment Interaction” in Thomson’s Institute of Scientific Information database, 1988–2007.
Formally, G×E occurs when genetic effects are conditional on environmental exposure. Shanahan and Hofer ( Shanahan & Hofer, 2005 ) have described the various forms of G×E; two of which are of particular interest to behavioral geneticists. First, G×E can arise when genetic effects are either triggered or amplified by exposure to a high-risk environment. Shanahan and Hofer termed this form of G×E Contextual Triggering, although most will recognize this as an instance of the Diathesis-Stress model. The Diathesis-Stress model was originally proposed to account for the development of schizophrenia ( Zubin & Spring, 1977 ), but is now applied to a wide range of psychopathological conditions. The most impressive support for this form of G×E in the behavioral genetic literature comes from a series of influential publications using the measured genotype approach by Caspi and colleagues. For example, Caspi et al. ( Caspi et al., 2002 ) reported that a functional polymorphism in the MAO-A gene was associated with increased violence only among those who had been maltreated in youth, while Caspi et al. ( Caspi et al., 2003 ) found that a functional polymorphism in the promoter of the serotonin transporter gene was associated with increased risk for depression only among those who had experienced high levels of psychological stress.
Shanahan and Hofer termed the second form of G×E Social Context as Social Control. This form of G×E refers to the existence of environments that suppress the expression of genetic effects. Typically, this suppression occurs because the environment limits individual choice. For example, Turkheimer and colleagues ( Turkheimer, Haley, Waldron, D'Onofrio, & Gottesman, 2003 ) showed, using the biometric approach to G×E, that genetic influences on intellectual achievement are suppressed in high poverty environments, presumably because impoverished environments provide limited opportunities to pursue and engage in intellectual pursuits. Similarly, Legrand and colleagues ( Legrand, Keyes, McGue, Iacono, & Krueger, 2008 ) using MCTFR data found that genetic effects on multiple indicators of adolescent disinhibited behavior were suppressed in rural as compared to urban environments, presumably because social monitoring of adolescent behavior, which is more likely to occur in rural as compared to urban environments, limits individual choice.
Although clearly on the ascendance, G×E approaches to human behavior are still at the initial stages of inquiry. Two general issues have been raised concerning the application of G×E approaches within behavioral genetics. First, are G×E effects replicable? It is well known that interactions are less statistically reliable than main effects ( Wahlsten, 1990 ), so that we might expect a priori that there will be difficulties in replicating G×E findings. Nonetheless, some G×E findings have accumulated an impressive record of replication. For example, the interaction between stress and the polymorphism in the promoter region of the serotonin transporter in the etiology of depression originally reported by Caspi et al. ( Caspi et al., 2003 ) has been replicated in the vast majority of published attempts to replicate ( Uher & McGuffin, 2008 ), while at least two independent studies have replicated Turkheimer et al.’s (2003) finding that the heritability of IQ is low in impoverished environments ( Harden, Turkheimer, & Loehlin, 2007 ; Rowe, Jacobson, & Van den Oord, 1999 ).
A second issue concerns the need for a theoretical framework to guide G×E research in human behavioral genetics. Shanahan and Hofer’s (2005) review suggests that there may generalizable G×E effects on behavior. Nonetheless, much of the current research on G×E is theoretically ad hoc, with minimal concern over whether G×E effects generalize across conceptually linked phenotypes and types of environmental exposure. Recently, we have begun to explore the general nature of G×E effects through analysis of MCTFR data. For example in the previously cited study by Legrand et al. (2008) , the moderating effect of urban/rural rearing on genetic influences was not specific to adolescent alcohol use, as had been indicated by previous research ( Rose, Dick, Viken, & Kaprio, 2001 ), but rather was characteristic of multiple indicators of adolescent disinhibitory psychopathology. Extending this line of inquiry with MCTFR data, Hicks et al. (2008) sought to determine whether the same form of G×E held across alternative forms of externalizing psychopathology and multiple measures of environmental adversity. For these conceptually related phenotypes and exposures there was strong evidence for a general form of G×E – in every case environmental adversity was found to amplify the magnitude of genetic influences on externalizing psychopathology.
Behavioral Genetics and Causal Inference
One of the greatest challenges in non-experimental disciplines is the drawing of causal inferences from observational data. Although psychologists have attempted to overcome the limitations of observational data through use of structural equation modeling (SEM), the typical application of SEM is burdened by untested assumptions and the extent to which this approach has fostered valid causal inference remains unclear ( Freedman, 2006 ). Alternatively, psychologists have attempted to take advantage of so-called “experiments of nature”, chance exposures that mimic experimental randomization even if they do not constitute true experiments. The difficulty with this latter strategy of course is that experiments of nature depend on serendipitous events that are outside the investigator’s control. Rutter ( Rutter, 2007 ; Rutter, Pickles, Murray, & Eaves, 2001 ) has recently shown, however, that several behavioral genetic research designs constitute experiments of nature and consequently provide unique opportunities for rigorous evaluation of causal hypotheses with observational data. We illustrate this aspect of contemporary behavioral genetic research with two examples of recent MCTFR research.
Adoption Studies and Causal Hypotheses About the Familial Transmission of Risk
One of the most salient features of common mental disorders is that they aggregate in families. Whether it is a mood disorder ( Currier, Mann, Oquendo, Galfalvy, & Mann, 2006 ; Weissman et al., 2006 ), an impulse-control disorder ( Faraone & Biederman, 1997 ), a substance use disorder ( Hicks, Krueger, Iacono, McGue, & Patrick, 2004 ; K.R. Merikangas et al., 1998 ), or an anxiety disorder ( K. R. Merikangas et al., 1998 ) the offspring of parents with a common mental disorder are at a substantially elevated risk of suffering the same disorder than are the offspring of unaffected parents. The familial transmission of psychopathology risk has implications for prevention, although to be effective the precise form prevention efforts take will likely depend on the mechanisms of familial transmission ( Collins, Murphy, & Bierman, 2004 ; Dierker, Avenevoli, Goldberg, & Glantz, 2004 ). Adoption studies can help to explicate the nature of these familial associations.
This approach can be illustrated by describing our recent study on the familial transmission of smoking ( M. Keyes, Legrand, Iacono, & McGue, 2008 ). There is a substantial body of research showing that the adolescent offspring of smoking parents are more much more likely to smoke than the adolescent offspring of non-smoking parents ( Jackson & Henriksen, 1997 ; Otten, Engels, van de Ven, & Bricker, 2007 ). Using the SIBS sample of adoptive and non-adoptive families, Keyes et al. (2008) sought to determine the extent to which parent smoking effects were genetically or environmentally mediated. As expected in non-adoptive families, parent smoking was significantly associated with excess risk of adolescent tobacco use; somewhat unexpectedly it was also associated with excess risk of alcohol use, marijuana use, and any disruptive disorder (the latter including oppositional defiant disorder, attention-deficit/hyperactivity disorder, and conduct disorder) in these families. A parallel analysis in adoptive families can help to implicate genetic and psychosocial mechanisms of these parent effects. Consistent with environmental contributions to transmission, adoptive parent smoking was significantly associated with increased adolescent risk of both tobacco and marijuana use, although the strength of these associations was smaller than in the non-adoptive families implicating genetic contributions to transmission as well. Alternatively, adoptive parent smoking was not significantly associated with excess risk of alcohol use or disruptive disorders, suggesting that genetic factors may underlie these parent smoking effects. This pattern of results thus implicates the existence of two mechanisms whereby parent smoking affects offspring risk ( Figure 4 ): 1) a primarily genetically-mediated pathway that conveys generalized vulnerability to disinhibitory psychopathology and substance abuse, and 2) a primarily environmentally-mediated pathway that conveys specific risk for smoking-related behavior. It would have been very difficult to uncover the dual nature of parent smoking risk without recourse to a natural experiment, like an adoption study.
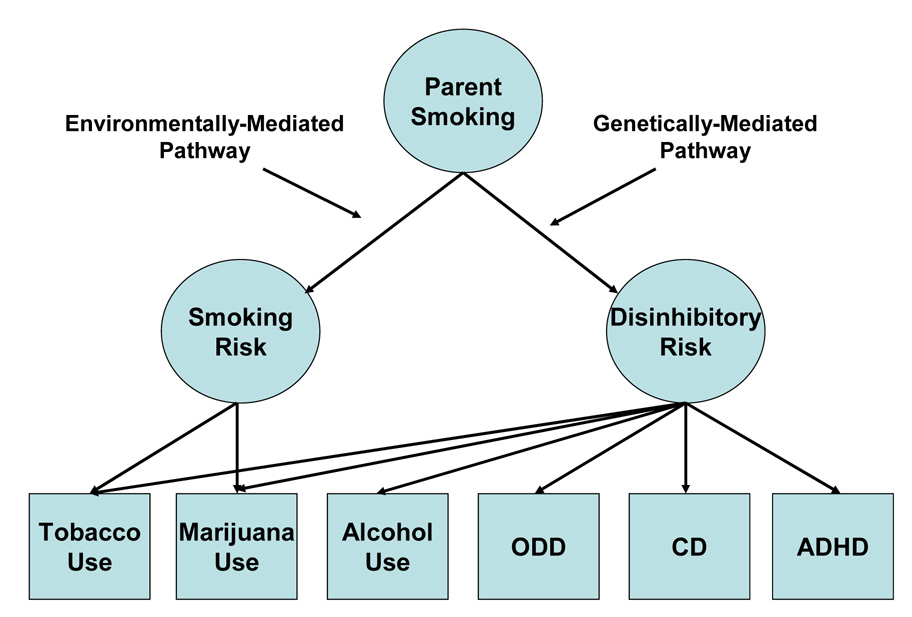
Dual pathway model for the effects of parent smoking on adolescent functioning. Model is based on the adoption study findings by Keyes et al. (2008) , which implicate the existence of a genetically-mediated pathway whereby parent smoking conveys generalized risk of disinhibitory psychopathology and substance abuse, and an environmentally mediated pathway whereby parent smoking conveys specific risk for adolescent smoking. ADHD = attention deficit/hyperactivity disorder, CD = conduct disorder, and ODD = oppositional defiant disorder.
Mendelian Randomization and a Novel Test of the Gateway Model
One of the most prominent models for the development of adolescent substance use is the Gateway Model, which was developed to account for the observation that adolescent substance use typically progresses from “softer” drugs like tobacco, alcohol and marijuana to “harder” drugs like cocaine and heroin ( Kandel, 2002 ; Kandel, Yamaguchi, & Chen, 1992 ). Specifically, the Gateway Model hypothesizes that the use of soft drugs directly increases the likelihood of the subsequent use of hard drugs by introducing adolescents to a drug using culture; that is, by serving as gateways. While there is little question that adolescent substance use typically progresses from softer to harder drugs, less certain is the causal basis for these associations - does soft drug use really causally contribute to hard drug use ( Golub & Johnson, 1998 ). This is a difficult question to address, since it would be unethical to undertake a randomized experiment in which adolescents are exposed to gateway substances. Nonetheless, the Gateway Model, if accurate, has profound implications for prevention efforts. We recently proposed that the method of Mendelian Randomization (MR) provided a natural experiment to test the gateway hypothesis ( Irons, McGue, Iacono, & Oetting, 2007 ).
MR takes advantage of the random assortment of alleles that occurs during meiosis that can, under certain circumstances, be used as an analog of a true experiment. Specifically, if a functional polymorphism exists in a gene that affects exposure (i.e., alcohol use for the purposes here), then the effects of exposure can be determined by investigating how inherited variation in this polymorphism is related to the behavioral or disease outcomes thought to be causally linked to exposure. The major limitation with the MR approach lies, of course, in identifying a polymorphism that affects exposure but is not otherwise likely to affect outcome. In the case of alcohol use such a polymorphism exists.
Figure 5 depicts the metabolic pathway involved in the elimination of alcohol. Alcohol is first converted to acetaldehyde by the enzyme alcohol dehydrogenase (ADH), and acetaldehyde is converted to acetate by the enzyme aldehyde dehydrogenase (ALDH). The second step is the rate limiting step in the metabolic breakdown of alcohol, and levels of acetaldehyde are associated with most of the dysphoric effects of drinking (e.g., dizziness, nausea). Consequently, genetic variation in the genes coding for the underlying enzymes, ALDH, and ADH, might be expected to affect not only the rate of alcohol elimination but also the likelihood an individual experiences dysphoria when drinking. Indeed, this is what has been found, with variation in the rate-limiting enzyme, ALDH, having the most powerful effect on drinking behavior. Specifically, a genetic variant in the gene that codes for ALDH2, the mitochondrial form of ALDH, codes for a deficient form of the enzyme. Consequently, individuals who inherit one or more copies of this allele are more much more likely to experience alcohol-induced dysphoria and flushing, and are much less likely to drink heavily or become alcoholic than individuals who did not inherit the deficient variant ( Harada, Agarwal, Goedde, Tagaki, & Ishikawa, 1982 ). Significantly, this polymorphism exists only among East Asians, where nearly 50% of the population carries at least one copy of the deficient allele ( Higuchi, Matsushita, Murayama, Tagaki, & Hayashida, 1995 ).
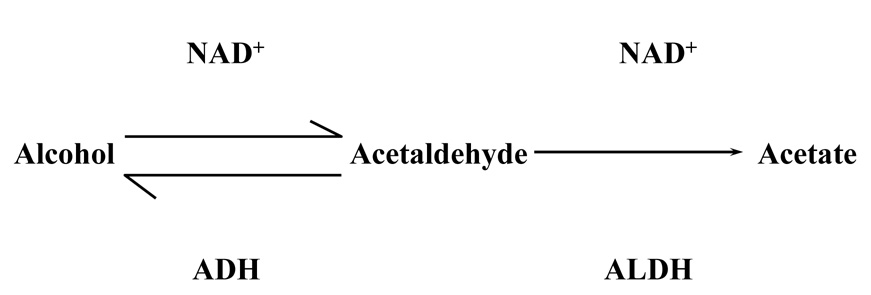
Primary metabolic pathway for the elimination of alcohol. The vast amount of ethanol consumed by mammals is metabolized in a two-step process in the liver. Alcohol is first converted to the intermediate product, acetaldehyde, by the enzyme alcohol dehydrogenase (ADH), which in turn is converted to acetate by the enzyme aldehyde dehydrogenase (ALDH) ‥
The ALDH2 polymorphism provides an ideal opportunity to use MR to test a key tenet of the gateway model. Genetic variation in ALDH2 is distributed randomly at meiosis and will affect exposure to alcohol. Moreover, ALDH2 is expressed only in liver, so it is highly unlikely that it would have any effect on behavioral outcomes other than its effect on drinking behavior. SIBS includes a large sample of adopted Asian-American adolescents, which Irons et al (2007) used to investigate the consequences of inheriting ALDH2 deficiency. As expected, inherited ALDH2 deficiency was significantly associated with reduced frequency of heavy drinking. That is, by chance some adolescents inherited a powerful protection against heavy drinking, but did inheriting this protection against drinking also result in their reduced use of other substances and delinquent behavior as the Gateway Model predicts? Contrary to this expectation, Irons et al. (2007) found no evidence for gateway effects. Those who inherited the deficient form of the ALDH2 allele were no less likely than those who did not to use tobacco, marijuana, or illicit drugs or to be delinquent and engage in antisocial acts. By indicating that the effects of ALDH2 deficiency did not extend beyond its primary effect on drinking behavior, this novel use of MR suggests that interventions that target adolescent alcohol use may have limited impact on other adolescent problem behaviors.
THE FUTURE OF BEHAVIORAL GENETIC RESEARCH
The possibility of technological breakthrough makes it risky, perhaps arrogant, to speculate about the future of any scientific discipline. Indeed, the focus of much behavioral genetic research today could not have been anticipated 20 or even 10 years ago. Nonetheless, while the precise nature of behavioral genetic research in the coming decades may be difficult to forecast, almost certain is that future progress will depend heavily on the discovery of the specific genes affecting behavior. Indeed, each of the three areas of contemporary behavioral genetic research highlighted above required knowledge of the specific genetic polymorphisms that underlie individual differences in behavior. Unfortunately, progress to date in identifying the specific genetic effects implied to exist in biometric analyses of behavioral data has been at best meager.
Several factors have contributed to the slow progress in identifying behaviorally-relevant genetic polymorphisms. It is important to recognize, however, that these problems are no different from those that have plagued gene identification efforts for a wide range of complex inherited diseases. Attempts to replicate initial positive findings in genetic association studies of behavioral as well as non-behavioral phenotypes have been much more likely to result in failure than success ( Hirschhorn, Lohmueller, Byrne, & Hirschhorn, 2002 ; Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001 ).
The association of the dopamine D2 receptor (DRD2) gene with alcoholism risk provides a useful example of the challenges of gene identification with complex behavioral phenotypes. In 1990, Blum and colleagues (1990) reported that 24 (69%) of 35 alcoholics but only 7 (20%) of 35 controls carried the A 1 allele at a restriction fragment site downstream of the DRD2 locus. This corresponds to an impressive odds ratio (OR) of 8.73 (95% confidence interval of 8.17 to 9.29), suggesting that genetic variation in DRD2 is a major contributor to alcoholism risk. Subsequent attempts to replicate Blum’s findings produced some positive replications ( Neiswanger, Hill, & Kaplan, 1995 ; Noble, 1998 ) but many more failures to replicate ( Gelernter et al., 1991 ; Kidd, 1993 ). While the inconsistent pattern of replication led some to dismiss the association as artifact ( Pato, Macciardi, Pato, Verga, & Kennedy, 1993 ), a recent meta-analysis of all published association studies suggests an alternative explanation ( Smith, Watson, Gates, Ball, & Foxcroft, 2008 ). Two findings from the meta-analysis are especially relevant to the discussion here. First, Smith et al. reported a pooled OR of 1.3, clearly much smaller than the original observation but nonetheless still statistically significant. Second, when the cumulative pooled OR was plotted against date of publication, it was found to decrease over time. That is, as more studies on the association of DRD2 with alcoholism were published, the pooled estimate of the size of the association decreased. This latter pattern is characteristic of what geneticists call the “Winner’s Curse” ( Goring, Terwilliger, & Blangero, 2001 ). An initial finding of significant genetic association is often based on a small sample in which multiple phenotypes and multiple genetic markers may have been investigated. To be significant in a small sample, and thus justify publication, the effect size will need to be large, and likely be overestimated because of multiple testing. Consequently, attempts to replicate, which are typically based on larger samples where there is less opportunity to capitalize on chance findings due to multiple testing, will report smaller and sometimes nonsignificant effects sizes. The importance of the DRD2/alcoholism saga is that it has shown us that: 1) genetic association studies will require large samples (in the thousands rather than the hundreds) to produce statistically reliable results; and 2) the effect of any single genetic locus is likely to be very small — ORs of 1.1 to 1.3 for categorical outcomes and < .5% of variance accounted for quantitative outcomes ( Plomin, Kennedy, & Craig, 2006 ).
Behavioral geneticists have adopted two strategies in an attempt to meet the challenges inherent to gene identification with behavioral conditions. Both strategies are being used at the MCTFR. The first is the endophenotype approach advocated by Gottesman and Gould ( Gottesman, II & Gould, 2003 ). The essence of this strategy is to identify phenotypes that are intermediate between primary gene product and the behavioral outcome of interest. The expectation is that because these intermediate or endophenotypes are more proximal to the biological substrate than clinical phenotypes, it will be easier to identify the genes that affect their expression. The endophenotype approach holds great promise and indeed appears to have been successfully applied in the addictions field ( Dick et al., 2006 ). Nonetheless, the validity of the basic rationale of this approach; namely, that the genetics of endophenotypes are simpler and therefore more amenable to molecular analysis than the genetics of clinical phenotypes; remains to be empirically tested ( Flint & Munafo, 2007 ).
The second strategy was first suggested in a highly influential 1996 publication by Risch and Merikangas ( Risch & Merikangas, 1996 ), who observed that the then predominant approach to gene identification, linkage analysis, had appeared to have reached its limit. As an alternative, they suggested that future progress would likely require the ability to systematically assay genetic variation across the whole genome by genotyping hundreds of thousands or maybe as many as one million genetic polymorphisms. In 1996, when investigations involving a couple of hundred genetic markers were considered path breaking, Risch and Merikangas’s proposal seemed fanciful. Remarkably, within a decade, breakthroughs in high-throughput genotyping that have enabled the efficient genotyping of up to 1,000,000 polymorphisms has made Risch and Merikangas’s proposal feasible and arguably the major approach currently in use for the genetic mapping of common disease.
Genome-wide association studies (GWASs) have resulted in the discovery of novel genetic variants affecting risk for a wide range of non-psychiatric diseases including macular degeneration, obesity, diabetes, breast cancer, and Crohn’s disease ( Iles, 2008 ). Although the number of published GWASs with psychiatric disorders is more limited, initial investigations of bipolar disorder ( Sklar et al., 2008 )and smoking ( Bierut et al., 2007 ) give reason to be optimistic that this approach will also lead to discovery in the behavioral domain. Nonetheless, it is important to recognize that GWASs do not represent a panacea for the challenges of gene identification in behavioral genetics ( Craddock, O'Donovan, & Owen, 2008 ). Existing GWASs suggest that the effect of any single genetic variant on complex phenotypes is likely to be small, much smaller than originally anticipated. A recent GWAS of adult height, for example, found significant associations with 20 different genetic variants, collectively these variants accounted for only 3% of the phenotypic variation in height ( Weedon et al., 2008 ). GWASs of behavioral traits will require massively large samples, which will likely only be feasible through collaborative efforts. Also, the GWAS approach is only applicable to the detection of common variants, and there is growing evidence that the genetic diathesis for some behavioral disorders, most notably autism, appears to be largely attributable to effect of multiple rare genetic mutations ( Burmeister, McInnis, & Zollner, 2008 ). Despite these limitations, the next 10 years is almost certain to see a large number of GWAS investigations of behavioral phenotypes.
This article began with an, admittedly rhetorical, question: The End of Behavior Genetics? The answer to this question may be yes for those with misplaced ideological misgivings or a fundamental misunderstanding of the nature and power of the reductionist paradigm ( Rutter et al., 2001 ). It is remarkable to reflect back on the past 100 years and consider the enormous impact behavioral geneticists have had on psychology. The way we think about mental illness, human intellectual achievement, and personality has been changed irrevocably by behavioral genetic research. But the behavioral genetic research agenda has never been simply about establishing the heritability of behavior. Rather, behavioral genetics has sought first to establish the importance of genetic influences; next to identify the specific nature of the factors that underlie those influences; and ultimately to understand how these genetic factors combine and/or interact with environmental factors to affect the development of complex behavioral phenotypes. The current vitality of the field owes in part to the power of its methodology but also to the as yet incomplete execution of this research agenda. Future progress will almost certainly depend on being able to identify the specific genetic polymorphisms that underlie behavior, a formidable task given the likely magnitude of specific genetic effects. Nonetheless, as we ponder the future of psychology over the next 100 years, it is inconceivable that behavioral genetics will not be at the center of many major breakthroughs in our understanding of the origins of human behavior, much like it has been at the center of many major breakthroughs over the past 100 years.
Acknowledgment
This paper is based in part on a presentation given at the 2008 biennial meeting of the Society for Research on Adolescence in Chicago Illinois. Research reported herein was supported in part from the following grants from the U.S. National Institutes of Health: AA09367 , AA11886, DA05147, and MH066140
- Ando J, Nonaka K, Ozaki K, Sato N, Fujisawa KK, Suzuki K, et al. The Tokyo Twin Cohort Project: Overview and initial findings. Twin Research and Human Genetics. 2006;9(6):817–826. doi: 10.1375/183242706779462480. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bioethics NCo. Genetics and human behaviour: The ethical context. London: Nuffield Council on Bioethics; 2002. [ Google Scholar ]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263(15):2055–2060. [ PubMed ] [ Google Scholar ]
- Bouchard TJ, McGue M. Familial studies of intelligence: A review. Science. 1981;212(4498):1055–1059. doi: 10.1126/science.7195071. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bouchard TJJ, Lykken DT, McGue M, Segal N, Tellegen A. Sources of human psychological differences: The Minnesota Study of Twins Reared Apart. Science. 1990;250:223–228. doi: 10.1126/science.2218526. [ DOI ] [ PubMed ] [ Google Scholar ]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nature Reviews Genetics. 2008;9(7):527–540. doi: 10.1038/nrg2381. [ DOI ] [ PubMed ] [ Google Scholar ]
- Busjahn A, Hur YM. Twin registries: An ongoing success story. Twin Research and Human Genetics. 2006;9(6):705–705. doi: 10.1375/183242706779462714. [ DOI ] [ PubMed ] [ Google Scholar ]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [ DOI ] [ PubMed ] [ Google Scholar ]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington HL, et al. Influence of life stress on depression: Moderation by polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chipuer HM, Rovine MJ, Plomin R. Lisrel modeling: Genetic and environmental influences on IQ revisited. Intelligence. 1990;14(1):11–29. [ Google Scholar ]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of Genearl Psychiatry. 1981;38(8):861–868. doi: 10.1001/archpsyc.1981.01780330019001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prevention Science. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Craddock N, O'Donovan MC, Owen MJ. Genome-wide association studies in psychiatry: lessons from early studies of non-psychiatric and psychiatric phenotypes. Molecular Psychiatry. 2008;13(7):649–653. doi: 10.1038/mp.2008.45. [ DOI ] [ PubMed ] [ Google Scholar ]
- Currier D, Mann MJ, Oquendo MA, Galfalvy H, Mann JJ. Sex differences in the familial transmission of mood disorders. Journal of Affective Disorders. 2006;95(1–3):51–60. doi: 10.1016/j.jad.2006.04.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- Devlin B, Daniels M, Roeder K. The heritability of IQ. Nature. 1997;388(6641):468–471. doi: 10.1038/41319. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dick DM, Jones K, Saccone N, Hinrichs A, Wang JC, Goate A, et al. Endophenotypes successfully lead to gene identification: Results from the collaborative study on the genetics of alcoholism. Behavior Genetics. 2006;36(1):112–126. doi: 10.1007/s10519-005-9001-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dierker LC, Avenevoli S, Goldberg A, Glantz M. Defining subgroups of adolescents at risk for experimental and regular smoking. Prevention Science. 2004;5(3):169–183. doi: 10.1023/b:prev.0000037640.66607.6b. [ DOI ] [ PubMed ] [ Google Scholar ]
- Eaves L, Martin N, Heath A, Schieken R, Meyer J, Silberg J, et al. Age changes in the causes of individual differences in conservatism. Behavior Genetics. 1997;27:121–124. doi: 10.1023/a:1025633307992. [ DOI ] [ PubMed ] [ Google Scholar ]
- Faraone SV, Biederman J. Familial transmission of attention deficit hyperactivity disorder and mood disorders. American Journal of Medical Genetics. 1997;74(6):569–569. [ Google Scholar ]
- Feldman MW, Lewontin RC. The heritability hang-up. Science. 1975;190(4220):1163–1168. doi: 10.1126/science.1198102. [ DOI ] [ PubMed ] [ Google Scholar ]
- Finkel D, McGue M. Sex differences and nonadditivity in heritability of the Multidimensional Personality Questionnaire scales. Journal of Personality and Social Psychology. 1997;72:929–938. doi: 10.1037//0022-3514.72.4.929. [ DOI ] [ PubMed ] [ Google Scholar ]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Freedman DA. Statistical models for causation - What inferential leverage do they provide? Evaluation Review. 2006;30(6):691–713. doi: 10.1177/0193841X06293771. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fromm-Reichmann F. Notes on the development of treatment of schizophrenia by psychoanalytic psychotherapy. Psychiatry. 1948;11:263–273. doi: 10.1080/00332747.1948.11022688. [ DOI ] [ PubMed ] [ Google Scholar ]
- Galton F. Heredity talent and character. II. Macmillan's Magazine. 1865;12:318–327. [ Google Scholar ]
- Galton F. Hereditary genius: An inquiry into its laws and consequences. London: MacMillan; 1869. [ Google Scholar ]
- Galton F. The history of twins, as a criterion for the relative powers of nature and nurture. Journal of the Anthropological Institute. 1876;5:391–406. [ Google Scholar ]
- Galton F. Inquiries into human faculty and its development. London: Macmillan; 1883. [ Google Scholar ]
- Gelernter J, O'Malley S, Risch N, Kranzler HR, Krystal J, Merikangas K, et al. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism [see comments] JAMA. 1991;266(13):1801–1807. [ PubMed ] [ Google Scholar ]
- Gillham NW. A life of Sir Francis Galton: From African exploration to the birth of eugenics. Oxford: Oxford University Press; 2001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Goddard HH. Human efficiency and levels of intelligence. Princeton, NJ: Princeton University Press; 1920. [ Google Scholar ]
- Golub AL, Johnson BD. Alcohol is not the gateway to hard drug abuse. Journal of Drug Issues. 1998;28(4):971–984. [ Google Scholar ]
- Goring HHH, Terwilliger JD, Blangero J. Large upward bias in estimation of locus-specific effects from genomewide scans. American Journal of Human Genetics. 2001;69(6):1357–1369. doi: 10.1086/324471. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gottesman II, Shields J. Schizophrenia and genetics: A twin study vantage point. New York: Academic Press; 1972. [ Google Scholar ]
- Harada S, Agarwal DP, Goedde HW, Tagaki S, Ishikawa B. Possible protective role against alcoholism for aldehyde dehydrogenase isozyme deficiency in Japan [letter] Lancet. 1982;2(8302):827. doi: 10.1016/s0140-6736(82)92722-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents' cognitive aptitude. Behavior Genetics. 2007;37(2):273–283. doi: 10.1007/s10519-006-9113-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Harris JR. The nurture assumption: Why children turn out the way they do. London: Bloomsbury; 1998. [ Google Scholar ]
- He MG, Ge J, Zheng YF, Huang WY, Zeng JW. The Guangzhou Twin Project. Twin Research and Human Genetics. 2006;9(6):753–757. doi: 10.1375/183242706779462561. [ DOI ] [ PubMed ] [ Google Scholar ]
- Heston LL. Psychiatric disorders in foster home reared children of schizophrenic mothers. British Journal of Psychiatry. 1966;112:819–825. doi: 10.1192/bjp.112.489.819. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Externalizing disorders account for the genetic and environmental overlap between nicotine dependence and major depression. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity increases genetic risk for externalizing disorders. 2008 doi: 10.1001/archgenpsychiatry.2008.554. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Higuchi S, Matsushita S, Murayama M, Tagaki S, Hayashida M. Alcohol and alcohol dehydrogenase polymorphisms and the risk of alcoholism. American Journal of Psychiatry. 1995;152(1219–1221) doi: 10.1176/ajp.152.8.1219. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genetics in Medicine. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hur YM, Shin JS, Jeong HU, Han JY. The South Korean Twin Registry. Twin Research and Human Genetics. 2006;9(6):838–843. doi: 10.1375/183242706779462606. [ DOI ] [ PubMed ] [ Google Scholar ]
- Iacono WG, McGue M, Krueger RF. Minnesota Center for Twin and Family Research. Twin Research and Human Genetics. 2007 doi: 10.1375/183242706779462642. in press. [ DOI ] [ PubMed ] [ Google Scholar ]
- Iles MM. What can genome-wide association studies tell us about the genetics of common disease? Plos Genetics. 2008;4(2) doi: 10.1371/journal.pgen.0040033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. [ DOI ] [ PubMed ] [ Google Scholar ]
- Irons DE, McGue M, Iacono WG, Oetting WS. Mendelian randomization: A novel test of the gateway hypothesis. Development and Psychopathology. 2007;19:1181–1195. doi: 10.1017/S0954579407000612. [ DOI ] [ PubMed ] [ Google Scholar ]
- Jackson C, Henriksen L. Do as I say: Parent smoking, antismoking socialization, and smoking onset among children. Addictive Behaviors. 1997;22(1):107–114. doi: 10.1016/0306-4603(95)00108-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kandel DB. Stages and pathways of drug involvement: Examining the gateway hypothesis. New York: Cambridge University Press; 2002. [ Google Scholar ]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kaprio J. Twin studies in Finland 2006. Twin Research and Human Genetics. 2006;9(6):772–777. doi: 10.1375/183242706779462778. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world - A developmental twin study of peer-group deviance. Archives of General Psychiatry. 2007;64(8):958–965. doi: 10.1001/archpsyc.64.8.958. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Keyes M, Legrand LN, Iacono WG, McGue M. Parent smoking and adolescent problem behavior: An adoption study of general and specific effects. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.08010125. in press. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Keyes MA, Iacono WG, McGue M. Early onset problem behavior, young adult psychopathology, and contextual risk. Twin Research and Human Genetics. 2007;10(1):45–53. doi: 10.1375/twin.10.1.45. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kidd KK. Associations of disease with genetic markers; deja vu all-over again. American Journal of Medical Genetics (Neuropsychaitric Genetics) 1993;48:71–73. doi: 10.1002/ajmg.1320480202. [ DOI ] [ PubMed ] [ Google Scholar ]
- Koenig LB, McGue M, Iacono WG. Stability and change in religiousness during emerging adulthood. Developmental Psychology. 2008;44(2):532–543. doi: 10.1037/0012-1649.44.2.532. [ DOI ] [ PubMed ] [ Google Scholar ]
- Koenig LB, McGue M, Krueger RF, Bouchard TJ. Genetic and environmental influences on religiousness: Findings for retrospective and current religiousness ratings. Journal of Personality. 2005;73(2):471–488. doi: 10.1111/j.1467-6494.2005.00316.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Legrand LS, Keyes MA, McGue M, Iacono WG, Krueger RF. Rural residency reduces the genetic influence on adolescent substance-use and rule-breaking behavior. Psychological Medicine. 2008 doi: 10.1017/S0033291707001596. in press. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lerner RM. Another nine-inch nail for behavioral genetics! Human Development. 2006;49(6):336–342. [ Google Scholar ]
- Li LM, Gao WJ, Lv J, Cao WH, Zhan SY, Yang HY, et al. Current status of the Chinese National Twin Registry. Twin Research and Human Genetics. 2006;9(6):747–752. doi: 10.1375/183242706779462651. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, et al. The Swedish Twin Registry in the third millennium: An update. Twin Research and Human Genetics. 2006;9(6):875–882. doi: 10.1375/183242706779462444. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Geneticae Medicae Et Gemellologiae. 1990;39(1):35–70. doi: 10.1017/s0001566000005572. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV, et al. Differential heritability of adult and juvenile antisocial traits. Arch Gen Psychiatry. 1995;52(11):906–915. doi: 10.1001/archpsyc.1995.03950230020005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Martin NG, Eaves LJ, Heath AC, Jardine R, Feingold LM, Eysenck HJ. Transmission of social attitudes. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4364–4368. doi: 10.1073/pnas.83.12.4364. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McCartney K, Harris MJ, Bernieri F. Growing up and growing apart: A developmental meta-analysis of twin studies. Psychological Bulletin. 1990;107(2):226–237. doi: 10.1037/0033-2909.107.2.226. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGue M, Bouchard TJ. Genetic and environmental influences on human behavioral differences. Annual Review of Neuroscience. 1997;21:1–24. doi: 10.1146/annurev.neuro.21.1.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGue M, Bouchard TJJ, Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: A life span perspective. In: Plomin R, McClearn GE, editors. Nature, nurture and psychology. Washington DC: American Psychological Association; 1993. pp. 59–76. [ Google Scholar ]
- McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: A longitudinal investigation. Developmental Psychology. 2005;41:971–984. doi: 10.1037/0012-1649.41.6.971. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry. 2005;162(6):1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGue M, Iacono WG. The adolescent origins of substance use disorders. International Journal of Methods in Psychiatric Research. 2008;17(S1):S30–S38. doi: 10.1002/mpr.242. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36(4):591–602. doi: 10.1007/s10519-006-9061-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, et al. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007;37(3):449–462. doi: 10.1007/s10519-007-9142-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGue M, Lykken DT. Genetic influence on risk of divorce. Psychological Science. 1992;3:368–373. [ Google Scholar ]
- Mednick SA, Gabrielli WF, Hutchings B. Genetic influences in criminal convictions: Evidence from an adoption cohort. Science. 1984;224(4651):891–894. doi: 10.1126/science.6719119. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mendlewicz J, Rainer JD. Adoption study supporting genetic transmission in manic-depressive illness. Nature. 1977;268(5618):327–329. doi: 10.1038/268327a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Merikangas KR, Stevens DE, Fenton B, Stolar M, O'Malley S, Woods SW, et al. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychological Medicine. 1998;28(4):773–788. doi: 10.1017/s0033291798006941. [ DOI ] [ PubMed ] [ Google Scholar ]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, et al. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mischel W. Introduction to personality. Austin, Tx: Holt, Rinehart and Winston; 1981. [ Google Scholar ]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [ DOI ] [ PubMed ] [ Google Scholar ]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer; 1992. [ Google Scholar ]
- Neiswanger K, Hill SY, Kaplan BB. Association and linkage studies of the TAQI A1 allele at the dopamine D2 receptor gene in samples of female and male alcoholics [see comments] American Journal of Medical Genetics. 1995;60(4):267–271. doi: 10.1002/ajmg.1320600402. [ DOI ] [ PubMed ] [ Google Scholar ]
- Noble EP. The D2 dopamine receptor gene: a review of association studies in alcoholism and phenotypes. Alcohol. 1998;16(1):33–45. doi: 10.1016/s0741-8329(97)00175-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Otten R, Engels R, van de Ven MOM, Bricker JB. Parental smoking and adolescent smoking stages: The role of parents' current and former smoking, and family structure. Journal of Behavioral Medicine. 2007;30(2):143–154. doi: 10.1007/s10865-006-9090-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pang ZC, Ning F, Unger J, Johnson CA, Wang SJ, Guo Q, et al. The Qingdao Twin Registry: A focus on chronic disease research. Twin Research and Human Genetics. 2006;9(6):758–762. doi: 10.1375/183242706779462859. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pato CN, Macciardi F, Pato MT, Verga M, Kennedy JL. Review of the putative association of dopamine D2 receptor and alcoholism: A metaanalysis. American Journal of Medical Genetics. 1993;48(2):78–82. doi: 10.1002/ajmg.1320480204. [ DOI ] [ PubMed ] [ Google Scholar ]
- Pinker S. The blank slate. New York: Penguin; 2002. [ Google Scholar ]
- Plomin R, Daniels D. Why are children in the same family so different from one another? Behavior and Brain Sciences. 1987;10:1–60. [ Google Scholar ]
- Plomin R, Kennedy JKJ, Craig IW. The quest for quantitative trait loci associated with intelligence. Intelligence. 2006;34(6):513–526. [ Google Scholar ]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;6:554–571. doi: 10.1375/136905202762342026. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Research and Human Genetics. 2006;9(6):941–949. doi: 10.1375/183242706779462895. [ DOI ] [ PubMed ] [ Google Scholar ]
- Richardson K, Norgate SH. A critical analysis of IQ studies of adopted children. Human Development. 2006;49(6):319–335. [ Google Scholar ]
- Risch NJ, Merikangas KR. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25(5):637–643. [ PubMed ] [ Google Scholar ]
- Rowe DC, Jacobson KC, Van den Oord E. Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development. 1999;70(5):1151–1162. doi: 10.1111/1467-8624.00084. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2(4):377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rutter M, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annual Review of Psychology. 2002;53:463–490. doi: 10.1146/annurev.psych.53.100901.135223. [ DOI ] [ PubMed ] [ Google Scholar ]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype => environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: Retrospect and prospect. Journal of Gerontology. 2005;60B:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sklar P, Smoller JW, Fan J, Ferreira MAR, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Molecular Psychiatry. 2008;13(6):558–569. doi: 10.1038/sj.mp.4002151. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Skytthe A, Kyvik K, Bathum L, Holm N, Vaupel JW, Christensen K. The Danish Twin Registry in the new millennium. Twin Research and Human Genetics. 2006;9(6):763–771. doi: 10.1375/183242706779462732. [ DOI ] [ PubMed ] [ Google Scholar ]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the Risk of alcohol depedency: A HuGE gene-disease association review. American Journal of Epidemiology. 2008;167(2):125–138. doi: 10.1093/aje/kwm281. [ DOI ] [ PubMed ] [ Google Scholar ]
- Teasdale TW, Owen DR. Heredity and familial environment in intelligence and educational-level: A sibling study. Nature. 1984;309(5969):620–622. doi: 10.1038/309620a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry. 2008;13(2):131–146. doi: 10.1038/sj.mp.4002067. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral and Brain Sciences. 1990;13(1):109-&. [ Google Scholar ]
- Watson JB. Behaviorism (revised edition) Chicago: University of Chicago Press; 1930. [ Google Scholar ]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nature Genetics. 2008;40(5):575–583. doi: 10.1038/ng.121. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. American Journal of Psychiatry. 2006;163(6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wender PH, Rosenthal D, Ketty SS, Schulsinger F, Welner J. Cross-fostering: A research strategy for clarifying the role of genetic and experimental factors in the etiology of schizophrenia. Archives of General Psychiatry. 1974;30:121–128. doi: 10.1001/archpsyc.1974.01760070097016. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zubin J, Spring B. Vulnerability: A new view of schizophrenia. Journal of Abnormal Psychology. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (722.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Celebrating a Century of Research in Behavioral Genetics
Affiliation.
- 1 Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK. [email protected].
- PMID: 36662387
- PMCID: PMC9922236
- DOI: 10.1007/s10519-023-10132-3
A century after the first twin and adoption studies of behavior in the 1920s, this review looks back on the journey and celebrates milestones in behavioral genetic research. After a whistle-stop tour of early quantitative genetic research and the parallel journey of molecular genetics, the travelogue focuses on the last fifty years. Just as quantitative genetic discoveries were beginning to slow down in the 1990s, molecular genetics made it possible to assess DNA variation directly. From a rocky start with candidate gene association research, by 2005 the technological advance of DNA microarrays enabled genome-wide association studies, which have successfully identified some of the DNA variants that contribute to the ubiquitous heritability of behavioral traits. The ability to aggregate the effects of thousands of DNA variants in polygenic scores has created a DNA revolution in the behavioral sciences by making it possible to use DNA to predict individual differences in behavior from early in life.
Keywords: Genome-wide association; Intelligence; Molecular genetics; Polygenic scores; Quantitative genetics.
© 2023. The Author(s).
Publication types
- Research Support, Non-U.S. Gov't
- Genetics, Behavioral*
- Genome-Wide Association Study*
- Multifactorial Inheritance / genetics
- Oligonucleotide Array Sequence Analysis
Grants and funding
- G19/2/MRC_/Medical Research Council/United Kingdom
- MR/M021475/1/MRC_/Medical Research Council/United Kingdom
- MR/V012878/1/MRC_/Medical Research Council/United Kingdom
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 09 February 2023
Behavioural genetics methods
- Emily A. Willoughby ORCID: orcid.org/0000-0001-7559-1544 1 ,
- Tinca J. C. Polderman ORCID: orcid.org/0000-0001-5564-301X 2 , 3 &
- Brian B. Boutwell 4 , 5
Nature Reviews Methods Primers volume 3 , Article number: 10 ( 2023 ) Cite this article
1895 Accesses
21 Citations
28 Altmetric
Metrics details
- Behavioural genetics
- Human behaviour
The question of why people show individual differences in their behaviours and capacities has intrigued researchers for centuries. Behaviour genetics offers us various methods to address this question. The answers are interesting for a range of research fields, varying from medicine to psychology, economics and neuroscience. Starting with twin and family studies in the late 1970s, the field of behaviour genetics has rapidly developed by applying molecular genetic techniques next to, and sometimes combined with, family data. The overarching conclusion at this point in time is that all measured human traits are to some extent heritable, and that many genetic variants, with each exerting a small effect, explain this heritability. Against this backdrop, we offer readers who might be less familiar with behaviour genetics a brief Primer on the topic. Sitting atop our list of goals is to be a resource for scholars interested in applying the widely useful techniques of the field to their particular specialty, regardless of what that might be.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Dissecting polygenic signals from genome-wide association studies on human behaviour
Beyond the factor indeterminacy problem using genome-wide association data
Maximizing the value of twin studies in health and behaviour
Turkheimer, E. Three laws of behavior genetics and what they mean. Curr. Dir. Psychol. Sci. 9 , 160–164 (2000).
Article Google Scholar
Harden, K. P. “Reports of my death were greatly exaggerated”: behavior genetics in the postgenomic era. Annu. Rev. Psychol. 72 , 37–60 (2021).
Barnes, J. C. et al. Demonstrating the validity of twin research in criminology. Criminology 52 , 588–626 (2014).
Friedman, N. P., Banich, M. T. & Keller, M. C. Twin studies to GWAS: there and back again. Trends Cognit. Sci. 25 , 855–869 (2021).
Polderman, T. J. et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47 , 702–709 (2015).
Chabris, C. F., Lee, J. J., Cesarini, D., Benjamin, D. J. & Laibson, D. I. The fourth law of behavior genetics. Curr. Dir. Psychol. Sci. 24 , 304–312 (2015).
Visscher, PeterM. et al. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet. 2 , e41 (2006).
Plomin, R., DeFries, J. C. & Loehlin, J. C. Genotype–environment interaction and correlation in the analysis of human behavior. Psychol. Bull. 84 , 309–322 (1997).
Scarr, S. & McCartney, K. How people make their own environments: a theory of genotype–environment effects. Child. Dev. 54 , 424 (1983).
Google Scholar
Fowler-Finn, K. D. & Boutwell, B. B. Using variation in heritability estimates as a test of G × E in behavioral research: a brief research note. Behav. Genet. 49 , 340–346 (2019).
Tucker-Drob, E. M. & Bates, T. C. Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychol. Sci. 27 , 138–149 (2016).
Loehlin, J., Corley, R., Reynolds, C. & Wadsworth, S. Heritability × SES interaction for IQ: is it present in US adoption studies? Behav. Genet. 52 , 1–8 (2022).
Heath, A. C., Kendler, K. S., Eaves, L. J. & Markell, D. The resolution of cultural and biological inheritance: informativeness of different relationships. Behav. Genet. 15 , 439–465 (1985).
Truett, K. R. et al. A model system for analysis of family resemblance in extended kinships of twins. Behav. Genet. 24 , 35–49 (1994).
Keller, M. C. et al. Modeling extended twin family data I: description of the Cascade model. Twin Res. Hum. Genet. 12 , 8–18 (2009).
Pettersson, E. et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol. Med. 49 , 1166–1173 (2019).
Lawlor, D. A., Tilling, K. & Davey Smith, G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45 , 1866–1886 (2016).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42 , 565–569 (2010).
Young, A. I. Solving the missing heritability problem. PLoS Genet. 15 , e1008222 (2019).
Young, A. I. Discovering missing heritability in whole-genome sequencing data. Nat. Genet. 54 , 224–226 (2022).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits’. Nat. Genet. 47 , 1236–1241 (2015).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 , 658–665 (2013).
Falconer, D. S. Introduction to quantitative genetics (Longman Group, 1960).
Falconer, D. S. & Mackay, T. F. C. Quantitative Genetics (Longman Group, 1996).
Martin, N. & Eaves, L. The genetical analysis of covariance structure. Heredity 38 , 79–95 (1977).
Neale, M. C. et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika 81 , 535–549 (2016).
Article MathSciNet MATH Google Scholar
Burt, S. A., McGue, M. & Iacono, W. G. Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: results from a cross-lagged monozygotic twin differences design. Dev. Psychol. 45 , 1752–1760 (2009).
Bartels, M., Boomsma, D. I., Hudziak, J. J., van Beijsterveldt, T. C. E. M. & van den Oord, E. J. C. G. Twins and the study of rater (dis)agreement. Psychol. Meth. 12 , 451–466 (2007); erratum 13 , 170 (2008).
Nivard, M. G., Middeldorp, C. M., Dolan, C. V. & Boomsma, D. I. Genetic and environmental stability of neuroticism from adolescence to adulthood. Twin Res. Hum. Genet. 18 , 746–754 (2015).
Visscher, P. M. et al. 10 years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 101 , 5–22 (2017).
Willoughby, E. & Lee, J. in The Cambridge Handbook of Intelligence and Cognitive Neuroscience (eds Barbey, A., Karama, S., & Haier, R.) 349–364 (Cambridge Univ. Press, 2021).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 9 , e1003348 (2013).
Plomin, R. & von Stumm, S. Polygenic scores: prediction versus explanation. Mol. Psychiatry 27 , 49–52 (2022).
Li, M. X. et al. A major gene model of adult height is suggested in Chinese. J. Hum. Genet. 49 , 148–153 (2004).
Roberts, D. F., Billewicz, W. Z. & McGregor, I. A. Heritability of stature in a West African population. Ann. Hum. Genet. 42 , 15–24 (1978).
Tarnoki, A. D., Tarnoki, D. L. & Molnar, A. A. Past, present and future of cardiovascular twin studies. Cor Vasa 56 , e486–e493 (2014).
Pechlivanis, S. et al. Risk prediction for coronary heart disease by a genetic risk score — results from the Heinz Nixdorf Recall study. BMC Med. Genet. 21 , 178 (2020).
Yang, R. et al. A healthy lifestyle mitigates the risk of heart disease related to type 2 diabetes: a prospective nested case–control study in a nationwide Swedish twin cohort. Diabetologia 64 , 530–539 (2021).
McGue, M., Osler, M. & Christensen, K. Causal inference and observational research: the utility of twins. Perspect. Psychol. Sci. 5 , 546–556 (2010).
Lemvigh, C. et al. The relative and interactive impact of multiple risk factors in schizophrenia spectrum disorders: a combined register-based and clinical twin study. Psychol. Med. https://doi.org/10.1017/S0033291721002749 (2021).
Squarcina, L., Fagnani, C., Bellani, M., Altamura, C. A. & Brambilla, P. Twin studies for the investigation of the relationships between genetic factors and brain abnormalities in bipolar disorder. Epidemiol. Psychiatr. Sci. 25 , 515–520 (2016).
Bouchard, T. J. & McGue, M. Genetic and environmental influences on human psychological differences. J. Neurobiol. 54 , 4–45 (2003).
Wilson, R. S. The Louisville twin study: developmental synchronies in behavior. Child. Dev. 54 , 298–316 (1983).
Bouchard, T. J., Lykken, D. T., McGue, M., Segal, N. L. & Tellegen, A. Sources of human psychological differences: the minnesota study of twins reared apart. Science. 250 , 223–228 (1990).
Article ADS Google Scholar
Bouchard, T. The Wilson effect: the increase in heritability of IQ with age. Twin Res. Hum. Genet. 16 , 923–930 (2013).
Deary, I. J., Johnson, W. & Houlihan, L. M. Genetic foundations of human intelligence. Hum. Genetics. 126 , 215–232 (2009).
Jang, K. L., Livesley, W. J. & Vernon, P. A. Heritability of the big five personality dimensions and their facets: a twin study. J. Pers. 64 , 577–591 (1996).
Tellegen, A. & Niels, G. W. Exploring personality through test construction: development of the multidimensional personality questionnaire. SAGE Handb. Pers. Theory Assess. 2 , 261–292 (2008).
Vukasović, T. & Bratko, D. Heritability of personality: a meta-analysis of behavior genetic studies. Psychol. Bull. 141 , 769–785 (2015).
Krueger, R. F., South, S., Johnson, W. & Iacono, W. The heritability of personality is not always 50%: gene–environment interactions and correlations between personality and parenting. J. Pers. 76 , 1485–1522 (2008).
Matteson, L. K., McGue, M. & Iacono, W. G. Shared environmental influences on personality: a combined twin and adoption approach. Behav. Genet. 43 , 491–504 (2013).
Howe, L. J. et al. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat. Genet. 54 , 581–592 (2022).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50 , 1112–1121 (2018).
Kong, A. et al. The nature of nurture: effects of parental genotypes. Science 359 , 424–428 (2018).
Belsky, D. W. et al. Genetic analysis of social-class mobility in five longitudinal studies. Proc. Natl Acad. Sci. USA 115 , E7275–E7284 (2018).
Bates, T. C. et al. Social competence in parents increases children’s educational attainment: replicable genetically-mediated effects of parenting revealed by non-transmitted DNA. Twin Res. Hum. Genet. 22 , 1–3 (2019).
Willoughby, E. A., McGue, M., Iacono, W. G., Rustichini, A. & Lee, J. J. The role of parental genotype in predicting offspring years of education: evidence for genetic nurture. Mol. Psychiatry 26 , 3896–3904 (2021).
Loehlin, J. C., Horn, J. M. & Willerman, L. in Intelligence, Heredity, and Environment (eds Sternberg, R. J., & Grigorenko, E. L.) 105–125 (Cambridge Univ. Press, 1997).
Cadoret, R. J. Adoption Studies. Alcohol Health Res. World 19 , 195–200 (1995).
Rhea, S. A., Bricker, J. B., Corley, R. P., DeFries, J. C. & Wadsworth, S. J. Design utility and history of the Colorado Adoption Project: examples involving adjustment interactions. Adopt. Q. 16 , 17–39 (2013).
Willoughby, E. A., McGue, M., Iacono, W. G. & Lee, J. J. Genetic and environmental contributions to IQ in adoptive and biological families with 30-year-old offspring. Intelligence 88 , 101579 (2021).
Baker, M. Reproducibility crisis? Nature 533 , 26 (2016).
Fanelli, D. Opinion: is science really facing a reproducibility crisis, and do we need it to? Proc. Natl Acad. Sci. USA 115 , 2628–2631 (2018).
Plomin, R., DeFries, J. C., Knopik, V. S. & Neiderhiser, J. M. Top 10 replicated findings from behavioral genetics. Perspect. Psychol. Sci. 11 , 3–23 (2016).
Chabris, C. F. et al. Most reported genetic associations with general intelligence are probably false positives. Psychol. Sci. 23 , 1314–1323 (2012).
Johnson, E. C. et al. No evidence that schizophrenia candidate genes are more associated with schizophrenia than noncandidate genes. Biol. Psychiatry 82 , 702–708 (2017).
Border, R. et al. No support for historical candidate gene or candidate gene-by-interaction hypotheses for major depression across multiple large samples. Am. J. Psychiatry 176 , 376–387 (2019).
Lin, X. Learning lessons on reproducibility and replicability in large scale genome-wide association studies. Harvard Data Sci. Rev. https://doi.org/10.1162/99608f92.33703976 (2020).
Røysamb, E., & Tambs, K. The beauty, logic and limitations of twin studies. Nor. Epidemiol . 26 , 35–46 (2016).
van Dongen, J., Slagboom, P. E., Draisma, H. H., Martin, N. G. & Boomsma, D. I. The continuing value of twin studies in the omics era. Nat. Rev. Genet. 13 , 640–653 (2012).
Wilson, S. et al. Minnesota Center for Twin and Family Research. Twin Res. Hum. Genet. 22 , 746–752 (2019).
Hindorff, L. A. et al. Prioritizing diversity in human genomics research. Nat. Rev. Genet. 19 , 175–185 (2017).
Mills, M. C. & Rahal, C. A scientometric review of genome-wide association studies. Commun. Biol. 2 , 1–11 (2019).
George, S., Duran, N. & Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health https://doi.org/10.2105/AJPH.2013.301706 (2014).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570 , 514–518 (2019).
Martin, A. R. et al. Human demographic history impacts genetic risk prediction across diverse populations. Am. J. Hum. Genet. 100 , 635–649 (2017).
Giannakopoulou, O. et al. The genetic architecture of depression in individuals of East Asian ancestry: a genome-wide association study. JAMA Psychiatry 78 , 1258–1269 (2021).
Meng, X. et al. Multi-ancestry GWAS of major depression aids locus discovery, fine-mapping, gene prioritisation, and causal inference. Preprint at bioRxiv https://doi.org/10.1101/2022.07.20.500802 (2022).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51 , 584–591 (2019).
Bigdeli, T. B. et al. Genetic effects influencing risk for major depressive disorder in China and Europe. Transl. Psychiatry 7 , e1074 (2017).
Bigdeli, T. B. et al. Contributions of common genetic variants to risk of schizophrenia among individuals of African and Latino ancestry. Mol. Psychiatry 25 , 2455–2467 (2020).
Hyttinen, V., Kaprio, J., Kinnunen, L., Koskenvuo, M. & Tuomilehto, J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 52 , 1052–1055 (2003).
Snieder, H. et al. HbA1c levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 50 , 2858–2863 (2001).
McAdams, T. A., Rijsdijk, F. V., Zavos, H. M. & Pingault, J. B. Twins and causal inference: leveraging nature’s experiment. Cold Spring Harb. Perspect. Med. 11 , a039552 (2021).
Alberg, A. J., Brock, M. V. & Samet, J. M. in Murray & Nadel’s Textbook of Respiratory Medicine 6th edn, Ch. 52, 927–939 (Saunders Elsevier, 2016).
Poulton, R., Moffitt, T. E. & Silva, P. A. The Dunedin multidisciplinary health and development study: overview of the first 40 years, with an eye to the future. Soc. Psychiatry Psychiatr. Epidemiol., 50 , 679–693 (2015).
Krüger, O., Korsten, P. & Hoffman, J. I. in J. Call, G. M. Burghardt, I. M. Pepperberg, C. T. Snowdon, & T. Zentall (Eds.), APA handbook of comparative psychology: Basic concepts, methods, neural substrate, and behavior (eds Call, J., Burghardt, G. M., Pepperberg, I. M., Snowdon, C. T. & Zentall, T.) 365–379 (American Psychological Association, 2017).
Turkheimer, E. & Harden, K. P. in Handbook of Research Methods in Social and Personality Psychology (eds Reis, H. T. & Judd, C. M.) 159–187 (Cambridge Univ. Press, 2014).
Okbay, A. et al. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat. Genet. 54 , 437–449 (2022).
Marouli, E. et al. Rare and low-frequency coding variants alter human adult height. Nature 542 , 186190 (2017).
Wainschtein, P. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat. Genet. 54 , 263–273 (2022).
Wang, Z., Gerstein, M. & Snyder, M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10 , 57–63 (2009).
Gupta, S. et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5 , 5748 (2014).
Pers, T.H. et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 6 , 5890 (2015).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11 , 1–19 (2015).
Neale, M. C. & Cardon, L. R. Methodology for Genetic Studies of Twins and Families . (Kluwer Academic/Plenum, 1992).
Vitaro, F., Brendgen, M. & Arseneault, L. Methods and measures: the discordant MZ-twin method: one step closer to the holy grail of causality. Int. J. Behav. Dev. 33 , 376–382 (2009).
Keller, M. C., Medland, S. E. & Duncan, L. E. Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behav. Genet. 40 , 377–393 (2010).
McAdams, T. A. et al. Revisiting the children-of-twins design: improving existing models for the exploration of intergenerational associations. Behav. Genet. 48 , 397–412 (2018).
Scarr, S. & Weinberg, R. A. The Minnesota Adoption Studies: genetic differences and malleability. Child. Dev. 54 , 260–267 (1983).
Murphy, K. et al. Twins Research Australia: a new paradigm for driving twin research. Twin Res. Hum. Genet. 22 , 438–445 (2019).
Otta, E. et al. The University of São Paulo Twin Panel: current status and prospects for Brazilian twin studies in behavioral research. Twin Res. Hum. Genet. 22 , 467–474 (2019).
Huang et al. The Chinese National Twin Registry: a unique data source for systems epidemiology of complex disease. Twin Res. Hum. Genet. 22 , 482–485 (2019).
Pedersen et al. The Danish Twin Registry: an updated overview. Twin Res. Hum. Genet. 22 , 499–507 (2020).
Bjerregaard-Andersen et al. The Guinea-Bissau Twin Registry update: a platform for studying twin mortality and metabolic disease. Twin Res. Hum. Genet. 22 , 554–560 (2019).
Gharipour et al. Isfahan Twins Registry (ITR): an invaluable platform for epidemiological and epigenetic studies: design and methodology of ITR. Twin Res. Hum. Genet. 22 , 579–582 (2019).
Ligthart et al. The Netherlands Twin Register: longitudinal research based on twin and twin-family designs. Twin Res. Hum. Genet. 22 , 623–636 (2019).
Rimfeld et al. Twins Early Development Study: a genetically sensitive investigation into behavioral and cognitive development from infancy to emerging adulthood. Twin Res. Hum. Genet. 22 , 508–513 (2019).
Pingault, J. B. et al. Using genetic data to strengthen causal inference in observational research. Nat. Rev. Genet. 19 , 566–580 (2018).
Lee, J. J. Correlation and causation in the study of personality. Eur. J. Personal. 26 , 372–412 (2012).
Vilhjámsson, B. J. et al. Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am. J. Hum. Genet. 97 , 576–592 (2015).
de Vlaming, R. & Groenen, P. J. F. The current and future use of ridge regression for prediction in quantitative genetics. BioMed. Res. Int. 2015 , 1–18 (2015).
Vattikuti, S., Lee, J. J., Chang, C. C., Hsu, S. D. H. & Chow, C. C. Applying compressed sensing to genome-wide association studies. GigaScience 3 , 10 (2014).
Lello, L. et al. Accurate genomic prediction of human height. Genetics 210 , 477–497 (2018).
Allegrini, A. G. et al. Genomic prediction of cognitive traits in childhood and adolescence. Mol. Psychiatry 24 , 819–827 (2019).
Download references
Acknowledgements
T.J.C.P. is supported by ZonMw grant 60-63600-98-834. Additionally, all of the authors thank the individuals who together provided incredibly constructive and useful feedback. All of these individuals contributed to the improvement of this Primer, and have their sincere gratitude for this.

Author information
Authors and affiliations.
Department of Psychology, University of Minnesota Twin Cities, Minneapolis, MN, USA
Emily A. Willoughby
Department of Clinical Developmental Psychology, Vrije Universiteit, Amsterdam, Netherlands
Tinca J. C. Polderman
Department of Child and Adolescent Psychiatry & Social Care, Amsterdam UMC, Amsterdam, Netherlands
School of Applied Sciences, University of Mississippi, University, MS, USA
- Brian B. Boutwell
John D. Bower School of Population Health, University of Mississippi Medical Center, Jackson, MS, USA
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (B.B.B. and T.J.C.P.); Experimentation (E.A.W.); Results (E.A.W.); Applications (E.A.W.); Reproducibility and data deposition (T.J.C.P.); Limitations and optimizations (B.B.B. and T.J.C.P.); Outlook (E.A.W. and B.B.B.); Overview of the Primer (T.J.C.P. and B.B.B.).
Corresponding author
Correspondence to Brian B. Boutwell .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Methods Primers thanks Thalia Eley, Elliott Rees, Lawrence Wilkinson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
A statistical method for evaluating how well a model fits the given data in which fit is penalized for the number of parameters estimated. In twin family studies, this term is often used to compare different possible models to determine which has the best fit.
A statistical method for evaluating model fit in which a penalty term is introduced for the number of parameters. This is a more explanatory tool that assesses the underlying data.
An extension of the extended twin family design that models covariances of twin pairs in addition to their siblings, spouses, parents and children. The Cascade model relaxes the assumptions of assortative mating and vertical transmission by allowing for the inclusion of latent phenotypes.
The simplest twin design in which pairs of monozygotic and dizygotic twins are compared for similarity on some phenotype, and the observed covariances are used to calculate the relative magnitudes of genetic and environmental sources of variance.
(DZ). Describes a pair of twins derived from two different sperm and two different ova who develop together in utero. DZ twins share approximately 50% of their DNA, similar to regular siblings.
The assumption that monozygotic and dizygotic twin pairs experience the same environmental factors.
A type of twin family design that models observed covariances using the relatives of twins in addition to the focal twin covariances for some variable.
( r GE). A phenomenon in which genes may influence individual variations in exposure to certain types of environment.
An interplay between genes and environments in which different genomes can cause individuals to respond differently to the same environmental exposure.
(MZ). Describes a pair of twins derived from a single sperm and ovum, who are therefore genetically identical.
The observation that the sum total of genetic variants from a genome-wide association study cannot completely explain the heritabilities of complex traits derived from twin family studies.
A type of twin family design that models observed covariances of the parents of twins in addition to the twins themselves.
The non-random placement of children based on traits that are similar between biological and adoptive families.
(PGS). A number generated from genome-wide association data that summarizes the estimated effect of a large number of summed genetic variants on a phenotype of interest.
An extension of the extended twin family design that models covariances of twin pairs in addition to their siblings, spouses, parents and children. The Stealth model relies on primary phenotypic assortment to model assortative mating, and on direct parent to offspring phenotypic transmission to model vertical transmission.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Willoughby, E.A., Polderman, T.J.C. & Boutwell, B.B. Behavioural genetics methods. Nat Rev Methods Primers 3 , 10 (2023). https://doi.org/10.1038/s43586-022-00191-x
Download citation
Accepted : 13 December 2022
Published : 09 February 2023
DOI : https://doi.org/10.1038/s43586-022-00191-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Educational attainment, crime, and causality: a population-wide sibling-based design.
- Steve G. A. van de Weijer
- Abigail Novak
Journal of Developmental and Life-Course Criminology (2024)
Same-Sex Relationships and Criminal Behavior: A Total Population Study in The Netherlands
- Sjoukje van Deuren
Archives of Sexual Behavior (2024)
The Challenges and Opportunities for Mental Health Twin Research in Nigeria
- Olakunle Ayokunmi Oginni
- Ayoyinka Ayorinde
- Kolawole Mosaku
Behavior Genetics (2024)
Genetic and environmental contributions to the subjective burden of social isolation during the COVID-19 pandemic
- Anita Kottwitz
- Bastian Mönkediek
- Jannis Hildebrandt
BMC Psychology (2023)
On the Usefulness of Behavior Genetics: Using Family Studies in Evolutionary Psychological Science to Improve Causal Inference and Sharpen Theory
- Dario Maestripieri
Adaptive Human Behavior and Physiology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Emerging Trends in Behavioral Genetic Studies of Child Temperament
Kimberly j saudino, lauren micalizzi.
- Author information
- Article notes
- Copyright and License information
Correspondence concerning this article should be addressed to Kimberly Saudino, Department of Psychological and Brain Sciences, Boston University, 64 Cummington Mall, Boston, MA, 02215; [email protected]
Kimberly J. Saudino, Department of Psychological and Brain Sciences, Boston University; Lauren Micalizzi, Department of Psychological and Brain Sciences, Boston University.
Issue date 2015 Sep.
In this article, we describe three emerging trends in the application of behavioral genetic methods to the study of temperament. The trends—using multiple methods to assess temperament, considering contextual influences on temperament, and evaluating the structure of temperament—have been well studied in the phenotypic literature, but adding a behavioral genetic perspective can enrich our understanding of temperament. We review recent behavioral genetic research in each of these areas and discuss its implications.
Keywords: temperament, behavioral genetics, individual differences
Behavioral genetics research indicates that genetic factors play a role in individual differences in children’s temperaments. In this work, researchers use genetically informative samples (e.g., twins or adoptive/nonadoptive siblings) to decompose the observed (i.e., phenotypic) variance of a temperament trait into genetic, shared, and nonshared environmental variance components. Heritability , the genetic effect size, is the proportion of phenotypic variance that can be attributed to genetic factors. If genetic influences are important to a trait or behavior, then behavioral similarity should covary with genetic relatedness (individuals who are more genetically similar should be more behaviorally similar). For example, most temperament theories assume that temperament has a biological or constitutional foundation ( 1 ); if this is the case, then genetically identical monozygotic twins who share all of their genes should be more similar in temperament than dizygotic twins who share, on average, only half of their segregating genes.
Shared environmental variance is familial resemblance that is not explained by genetic variance and comprises environmental influences that are shared by family members, such as family demographics, one’s rearing neighborhood, shared friends, or even the number of TVs or books in the house. If shared environments are important to individual differences in temperament, they should enhance the similarity of family members. Nonshared environmental variance is a residual variance that includes environmental influences that are unique to each individual. These unique environmental influences make members of the same family different from one another. Possible sources of nonshared environmental variance include differential parental treatment; relationships with friends, peers, and teachers; and nonsystematic factors such as accidents, illness, and measurement error ( 2 ).
Twin, adoption, and twin/sibling studies yield consistent evidence of genetic influences on most dimensions of temperament in early childhood, middle childhood, and adolescence. Heritability estimates range from .20 to .60, suggesting that genetic differences among individuals account for approximately 20 to 60 percent of the variability of temperament within a population ( 3 ). However, contemporary behavioral genetic studies rarely focus on heritability estimates because whether a given temperament trait is heritable is not usually the most interesting question. In this article, we describe three emerging trends in behavioral genetic studies of temperament that go beyond simple heritability estimates and may change substantially how we think about genetic and environmental influences on temperament, and perhaps temperament more generally.
Taking a Multimethod Approach
Although behavioral-genetics researchers typically assess temperament by using parent rating measures, in recent years parent ratings have been complemented by observational or lab-based measures. Different methods are thought to tap the same underlying constructs, but this is an empirical question that researchers in behavioral genetics can examine. Using many measures within the same group allows researchers to explore the extent to which different methods of assessing temperament are influenced by the same genetic and environmental factors. They can do this by using multivariate behavioral genetic analyses that explore genetic and environmental contributions to the covariance between multiple methods rather than the variance of each measure considered separately.
Recent research suggests that the covariance between different methods of assessing temperament is primarily due to overlapping genetic effects, but some genetic effects are also method-specific. For example, in a study of toddlers, the genetic correlation ( rG ) indexing the degree of genetic overlap between parent ratings and observational measures of inhibitory control was only moderate ( rG =. 47) indicating genetic effects unique to each measure ( 4 ). In addition, parent ratings, but not observed inhibitory control, showed substantial shared environmental influences.
Similar findings have emerged from a study of behavioral inhibition/shyness across toddlerhood: Multivariate models fit to parent and observer data at 14, 20, 24, and 36 months suggest that the two methods tap a common phenotype that is highly heritable, but that parents’ ratings are also subject to unique genetic influences ( 5 ). Positive affect may be an exception to the finding of novel genetic variance for parent ratings. The genetic correlation between observed smiling and laughter in the lab and parent-rated positive affect in 2-year-old twins was 1.0, indicating that the same genetic effects influenced the two measures ( 6 ). In contrast, shared environmental influences were unique to parent ratings and accounted for most of the variance (58 percent). Despite the differences across dimensions, in all studies, the phenotypic correlation between parent and observer ratings was entirely due to genetic effects that covaried across methods (i.e., environmental influences did not covary).
These findings may be limited because the different methods were used in different situations (e.g., parent ratings in the home and observations in the lab). A twin study of toddlers’ activity level assessed with multiple measures within the same situation ( 7 ) offers a stronger test of method-specific genetic effects, finding modest overlap between the factors ( rG = .38) that influenced actigraph and parents’ ratings of activity in the home. The findings suggest that both measures were genetically influenced, but the genetic effects on each measure were largely independent of each other. Again, although genetic covariance was modest, only these overlapping genetic influences contributed to the phenotypic correlation between measures.
These multimethod behavioral genetic studies indicate that genetic factors contribute both to the agreement and disagreement between different methods of assessing temperament. To the extent to which methods converge, it is due to the fact that they are tapping the same underlying genetic effects. However, agreement across methods is typically low, indicating that different methods are influenced by different factors ( 8 ). Behavioral genetics research reveals that these differences between methods arise due to both genetic and environmental influences.
Exploring Contextual Influences on Temperament
A second recent trend in behavioral genetics studies of temperament involves considering the effects of specific environments on the etiology of individual differences in temperament. Research on contextual influences has taken two approaches. The first examines within-individual context-specific effects by assessing children’s temperament across multiple situations and evaluates the extent to which the same genetic and environmental factors operate across situations. The second involves across-individual contextual effects and examines measured environments as modifiers of genetic and environmental influences on temperament. Both provide unique perspectives on the interplay between genes and the environment.
Within-Individual Contextual Effects
The within-individual approach asks if genetic and environmental influences on temperament change as the individual moves from situation to situation. To control for possible method effects, the same measures of temperament must be used across situations. Twin studies of shyness and activity level illustrate situation-specific genetic effects. In studies on observed shyness in infants in the home and the laboratory, researchers found substantial genetic overlap ( rG = .81) across the two situations but genetic effects specific to the home situation ( 9 ). Moreover, in a separate study of activity level in toddler twins assessed by actigraphs in home and laboratory test and play situations, results were similar ( 10 ). Genetic correlations across situations were substantial, ranging from .68 between the home and each laboratory situation to 1.0 between the laboratory test and play situations. Despite these considerable cross-situational genetic effects, genetic variance also was specific to the home environment. Approximately half of the genetic effects on activity in the home were independent of the genetic effects that influenced activity in the laboratory. The finding that the same genetic factors operated across the lab test and play situations mirrors results from a study of school-aged twins; in that study, a genetic correlation of 1.0 was reported between actigraph-assessed activity during cognitive testing and a 25-minute rest break ( 11 ). The novel genetic effects for the home but not for discrete situations within the lab illustrate that home-based measures provide additional information about temperament that might not be captured in a more artificial setting.
Contextual effects may be even subtler. The activity level of 5-month-old twins assessed while viewing televised sequences of neutral and happy facial expressions were differentially heritable depending on whether the actor was the mother or an unfamiliar female stranger ( 12 ). In the context of an unfamiliar female, genetic factors accounted for approximately 20 percent of the variance in activity level in both the neutral and happy conditions. The remaining variance was due to nonshared environmental influences. In contrast, when the same infants viewed the facial expressions of the mother (both neutral and happy), individual differences in activity were due solely to the environment, with shared environmental influences explaining 14 to 23 percent of the variance. Although not as robust, a similar pattern of modest genetic influences in the unfamiliar, but not the familiar, context emerged for social gaze and gaze aversion. Thus, even though the physical situation or tasks did not differ, the etiology of temperamental dimensions was not the same in the context of different actors.
Across-Individual Contextual Effects
Rather than looking at short-term situational change and the genetic and environmental overlap across situations within individuals, the second approach considers more enduring environments and tests for differences in the magnitude of genetic and environmental influence across individuals who experience varying levels of a measured environment. Behavioral genetics researchers refer to this as a genotype-environment interaction (GxE). The handful of studies exploring environmental moderators of genetic and environmental influences in children’s temperament provides novel evidence of the importance of environmental influences on individual differences in temperament.
Parenting and global aspects of the home environment moderate genetic and environmental influences on temperament. Genetic factors accounted for most of the variance in anger proneness for toddler twins who experienced much maternal negativity, but shared environmental factors accounted for most of the variance for twins who experienced less maternal negativity ( 13 ). Similarly, in middle childhood, both surgency/extraversion and effortful control were more heritable in homes that were more chaotic, and negative affectivity was more heritable in homes that had less optimal physical environments (i.e., crowded or unsafe; 14). In children, temperament may be more heritable under adverse environments, but in adolescent twins, the opposite pattern emerged. Genetic effects on both positive and negative emotionality were greater for those adolescents who rated their relationship with their parent as characterized by high levels of regard/warmth ( 15 ). At lower levels of regard/warmth, genetic influences diminished and the relative influence of the nonshared environment increased. Conflict within the parent-child relationship also moderated genetic influences on negative emotionality; as conflict increased, the relative impact of genetic influences decreased and the importance of the shared environment increased.
Although the direction of effects is inconsistent across studies of GxE interactions in children and adolescents, this work suggests a dynamic interplay between the environment and sources of variation in temperament that might be more satisfying to developmentalists who are often dismayed by the lack of familywide environmental influences on temperament. Shared environmental influences may be important for some individuals but may not be apparent in basic twin analyses because the analyses represent average estimates of genetic and environmental effects collapsing across all levels of unmeasured contexts ( 15 ).
Examining the Structure of Temperament
Until recently, little research has considered the structure of temperament from a behavioral genetics perspective. This may reflect the field of temperament more generally because, unlike in adult personality where there is general agreement about structure, researchers tend to disagree about the basic units of temperament ( 16 ). Most behavioral genetics research on temperament has been at the level of lower-order dimensions (e.g., activity level); however, as a hierarchical organization of temperament has become more prominent in the phenotypic literature, researchers have become more interested in genetic and environmental influences on individual differences in higher-order factors of temperament such as surgency/extraversion, negative affectivity, and effortful control. Twin studies of temperamental effortful control in toddlers ( 17 ), school-aged children ( 14 , 18 – 20 ), and young adults ( 21 ) consistently find substantial genetic and negligible shared environmental influences, whether assessed via parent report, behavioral observations, or self-report. When rated by parents, surgency/extraversion shows a similar pattern of significant genetic effects and nonsignficant shared environmental influences ( 14 , 17 , 19 , 20 ), but observer ratings suggest that both genetic and shared environmental influences contribute to familial resemblance in surgency/extraversion ( 22 , 23 ). Parent ratings of negative affectivity also show a consistent pattern of significant genetic influences across age, but evidence for shared environmental influences is mixed—even within the same sample ( 17 , 19 , 20 ). For example, shared environmental influences have been found for fathers’ ratings, but not mothers’ ratings ( 21 ), reminding us once more that methods matter.
These findings of genetic and environmental influences on higher-order temperament dimensions do not address fundamental questions regarding the structure of temperament. To what extent do the lower-order traits that load on a higher-order dimension share common genetic and environmental underpinnings? Multivariate analyses of the genetic and environmental overlap between lower-order traits can provide clues to whether the phenotypic structure of temperament reflects the underlying genetic structure. Although researchers have not examined this in children, a study of effortful control in adults suggests that this might be the case. The genetic correlations between subscales of effortful control ranged from .64 between inhibitory control and activation control to .93 between inhibitory control and attentional control, indicating that the three subscales are largely influenced by the same genetic factors ( 21 ). Environmental correlations (nonshared) were only moderate, suggesting genetic coherence between the dimensions that constitute the higher-order dimension of effortful control. At issue is whether a similar pattern will emerge for other higher-order dimensions (e.g., negative affect or surgency/extraversion) and in younger samples.
Genetic influences also covary between separate higher-order dimensions, although more modestly. The genetic correlations among positive affect/surgency, negative affect, and effortful control in middle childhood ranged from .17 to .51 ( 19 ). In contrast to these modest overlapping genetic influences, the nonshared environmental influences overlapped completely across all three dimensions and there was considerable shared environmental covariance ( rC = .89) between negative affect and effortful control. The genetic and environmental overlap between lower-order dimensions that load on different higher-order temperaments show a similar pattern. The genetic correlations among approach/positive anticipation and frustration/anger ( 24 ), task persistence and frustration/anger ( 25 ), and anger and inhibitory control ( 26 ) were, at best, only moderate, whereas the correlations between environmental factors across dimensions were substantial. More research is needed, but these findings of greater genetic convergence within, than between, higher-order dimensions are consistent with theories of temperament that propose that separate, but interrelated, neural substrates underlie higher-order temperament dimensions ( 27 ).
Implications
The behavioral genetic findings we have reviewed are relevant to researchers who study temperament from a phenotypic perspective. The multimethod findings highlight important issues regarding the measurement of temperament. The fact that different measures of the same temperament dimension have different etiologies means that researchers should not assume that all measures of temperament are interchangeable. Alternatively, a lack of agreement across methods may not simply reflect measurement error (i.e., measure-specific genetic and shared environmental effects are independent of measurement error). Findings with one method may not generalize to another because different methods tap different processes. Therefore, it is not surprising to find that different methods yield different developmental patterns (e.g., 28 ) or associations with developmental outcomes ( 26 , 29 ). Relying on a single assessment method may not allow a full understanding of temperament and the factors that underlie variations in temperament across children. More generally, these findings have important implications for issues of replication, a topic that is becoming increasingly relevant in psychology. When measures differ across studies, failures to replicate may reflect differences in the underlying etiologies of methods assumed to assess the same trait.
Similarly, findings of context-specific genetic effects suggest that different situations provide different views of temperament. Diverse situations likely place different demands on the individual, elicit different behaviors, and consequently, engage different genetic influences on processes relevant to each context. Because the genetic and environmental influences that underlie individual differences in temperament may vary across situation, researchers should consider carefully the context in which temperament is assessed. Moreover, these findings provide a unique perspective on situations: Behavioral differences across situations can have a genetic basis and consequently, contextual differences are not necessarily environmental in origin.
GxE analyses permit a more nuanced understanding of the etiology of temperament, and reveal novel evidence of direct and indirect environmental influences on temperament. Shared environments may directly influence individual differences in temperament under certain contexts (e.g., when the child experiences more positive parenting). Environments can also indirectly influence temperament by modulating the expression of children’s genetically influenced temperaments, thereby potentially enhancing or diminishing children’s genetically based vulnerability for later emotional and behavioral problems. This adds an interesting twist to the notion of differential sensitivity, which suggests that some temperaments may be more responsive to the environment ( 30 ). The GxE findings we have reviewed suggest that temperamental differential susceptibility might arise from specific environmental experiences.
Behavioral genetics research can also inform the structure of temperament. Factor analytic studies of temperament have indicated a hierarchical structure of temperament ( 31 ). The use of genetically informative samples can reveal the extent to which this structure is based in biology. Although research on the structure of temperament has just begun, findings hint that, as is the case with the study of adult personality ( 32 ), the phenotypic architecture of temperament reflects its underlying genetic structure. Facets that load on the same higher-order dimension are largely influenced by the same genetic factors, whereas different dimensions are more genetically distinct. These results provide early evidence that dimensions of temperament are genetically coherent, and that the structure of temperament has a biological basis and is not simply a statistical phenomenon.
Conclusions
The research we have described here reveals that behavioral genetic research has provided powerful insights into issues related to the measurement of temperament, contextual effects and environmental moderators of genetic effects on temperament, and the underlying structure of temperament. These are just a few areas in which our knowledge of temperament can be enriched by applying a behavioral genetics approach. Clearly, behavioral genetics research has much more to offer developmentalists than simple heritability estimates.
Acknowledgments
This work was supported in part by grant #R01HD068435 (to Dr. Saudino) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
- 1. Shiner RL, Buss KA, McClowry SG, Putnam SP, Saudino KJ, Zentner M. What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. Child Development Perspectives. 2012;6:436–444. [ Google Scholar ]
- 2. Plomin R, Chipuer HM, Neiderhiser JM. Behavioral genetic evidence for the importance of nonshared environment. In: Hetherington EM, Reiss D, Plomin R, editors. Separate social worlds of siblings: Importance of nonshared environment on development. Hillsdale, NJ: Lawrence Erlbaum; 1994. pp. 1–31. [ Google Scholar ]
- 3. Saudino KJ, Wang M. Quantitative and molecular genetic studies of temperament. In: Zentner M, Shiner R, editors. The handbook of temperament. New York, NY: Guilford; 2012. pp. 315–346. [ Google Scholar ]
- 4. Gagne JR, Saudino KJ. Wait for it! A twin study of inhibitory control in early childhood. Behavior Genetics. 2010;40:327–337. doi: 10.1007/s10519-009-9316-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Smith AK, Rhee SH, Corley RP, Friedman NP, Hewitt JK, Robinson JL. The magnitude of genetic and environmental influences on parental and observational measures of behavioral inhibition and shyness in toddlerhood. Behavior Genetics. 2012;42:764–777. doi: 10.1007/s10519-012-9551-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Wang M, Saudino KJ. Environmental influences on positive affect: Different measures, different results. Paper presented at the 70th Biennial Meetings of the Society for Research in Child Development; Seattle, WA.. 2013. Apr, [ Google Scholar ]
- 7. Saudino KJ. Do different measures tap the same genetic influences? A multi-method study of activity level in young twins. Developmental Science. 2009;12:626–633. doi: 10.1111/j.1467-7687.2008.00801.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Saudino KJ. The development of temperament from a behavioral genetics perspective. Advances in Child Development and Behavior. 2009;37:201–231. doi: 10.1016/s0065-2407(09)03705-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Cherny SS, Saudino KJ, Fulker DW, Plomin R, Corley RP, DeFries JC. The development of observed shyness from 14 to 20 months: Shyness in context. In: Emde RN, Hewitt JK, editors. Infancy to early childhood: Genetic and environmental influences on developmental change. New York, NY: Oxford University Press; 2001. pp. 269–282. [ Google Scholar ]
- 10. Saudino KJ, Zapfe JA. Genetic influences on activity level in early childhood: Do situations matter? Child Development. 2008;79:930–943. doi: 10.1111/j.1467-8624.2008.01168.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Wood AC, Saudino KJ, Rogers H, Asherson P, Kuntsi J. Genetic influences on mechanically-assessed activity level in children. Journal of Child Psychology and Psychiatry. 2007;48:695–702. doi: 10.1111/j.1469-7610.2007.01739.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Soussignan R, Boivin M, Girard A, Pérusse D, Liu X, Tremblay RE. Genetic and environmental etiology of emotional and social behaviors in 5-month-old infant twins: Influence of the social context. Infant Behavior and Development. 2009;32:1–9. doi: 10.1016/j.infbeh.2008.09.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Ganiban JM, Saudino KJ. Associations between child anger and parenting during toddlerhood: Underlying mechanisms. Behavior Genetics. 2011;41:908. [ Google Scholar ]
- 14. Lemery-Chalfant K, Kao K, Swann G, Goldsmith HH. Childhood temperament: Passive gene-environment correlation, gene-environment interaction, and the hidden importance of the family environment. Development and Psychopathology. 2013;25:51–63. doi: 10.1017/S0954579412000892. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Krueger RF, South S, Johnson W, Iacono W. The heritability of personality is not always 50%: Gene-environment interactions and correlations between personality and parenting. Journal of Personality. 2008;76:1485–1522. doi: 10.1111/j.1467-6494.2008.00529.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Shiner RL, DeYoung CG. The structure of temperament and personality traits: A developmental perspective. In: Zelazo P, editor. Oxford handbook of developmental psychology. New York, NY: Oxford University Press; 2013. pp. 113–141. [ Google Scholar ]
- 17. Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: Expended content, stronger genetic evidence, new evidence for the importance of environment. Developmental Psychology. 1997;33:891–905. doi: 10.1037//0012-1649.33.6.891. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Lemery-Chalfant K, Doelger L, Goldsmith HH. Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant and Child Development. 2008;17:365–385. doi: 10.1002/icd.581. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Allan NP, Mikolajewski AJ, Lonigan CJ, Hart SA, Taylor J. Examining the etiological associations among higher-order temperament dimensions. Journal of Research in Personality. 2014;48:51–60. doi: 10.1016/j.jrp.2013.11.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Mullineaux PY, Deater-Deckard K, Petrill SA, Thompson LA, DeThorne LS. Temperament in middle childhood: A behavioral genetic analysis of fathers’ and mothers’ reports. Journal of Research in Personality. 2009;43:737–746. doi: 10.1016/j.jrp.2009.04.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Yamagata S, Takahashi Y, Kijima N, Maekawa H, Ono Y, Ando J. Genetic and environmental etiology of effortful control. Twin Research and Human Genetics. 2005;8:300–306. doi: 10.1375/1832427054936790. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Roisman GI, Fraley RC. The limits of genetic influence: A behavior-genetic analysis of infant-caregiver relationship quality and temperament. Child Development. 2006;77:1656–1667. doi: 10.1111/j.1467-8624.2006.00965.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Wang Z, Chen N, Petrill SA, Deater-Deckard K. Observed personality in childhood: Psychometric and behavioral genetic evidence of two broad personality factors. European Journal of Personality. 2013;27:96–105. doi: 10.1002/per.1886. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Deater-Deckard K, Beekman C, Wang Z, Kim J, Petrill S, Thompson L, DeThorne L. 201Approach/positive anticipation, frustration/anger, and overt aggression in childhood. Journal of Personality. 78:991–1010. doi: 10.1111/j.1467-6494.2010.00640.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Deater-Deckard K, Petrill SA, Thompson LA. Anger/frustration, task persistence, and conduct problems in childhood: A behavioral genetic analysis. Journal of Child Psychology and Psychiatry. 2007;48:80–87. doi: 10.1111/j.1469-7610.2006.01653.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Gagne JR, Goldsmith HH. A longitudinal analysis of anger and inhibitory control in twins from 12 to 36 months of age. Developmental Science. 2011;14:112–124. doi: 10.1111/j.1467-7687.2010.00969.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology, Vol. 3.: Social, emotional, and personality development. 6. New York, NY: Wiley; 2006. pp. 99–166. [ Google Scholar ]
- 28. Saudino KJ. Sources of continuity and change in activity level in early childhood. Child Development. 2012;83:266–281. doi: 10.1111/j.1467-8624.2011.01680.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Gagne JR, Saudino KJ, Asherson P. The genetic etiology of inhibitory control and behavior problems at 24 months of age. Journal of Child Psychology and Psychiatry. 2011;52:1155–1163. doi: 10.1111/j.1469-7610.2011.02420.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspectives in Psychological Science. 2009;4:345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behavior and Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Yamagata S, Suzuki A, Ando J, Ono Y, Kijima N, Yoshimura K, Jang KL. Is the genetic structure of human personality universal? A cross-cultural twin study from North America, Europe, And Asia. Journal of Personality and Social Psychology. 2006;90:987–998. doi: 10.1037/0022-3514.90.6.987. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (64.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
COMMENTS
4 days ago · Behavioural genetics is the interdisciplinary effort to establish causal links between genes and animal (including human) behavioural traits and neural mechanisms.
The first human behavioral genetic research on intelligence and mental illness began in the 1920s, when environmentalism (the theory that behaviour is a result of nongenetic factors such as various childhood experiences) became popular and before Nazi Germany’s abuse of genetics made the notion of hereditary influence abhorrent.
The start of behaviour genetics as a well-identified field was marked by the publication in 1960 of the book Behavior Genetics by John L. Fuller and William Robert (Bob) Thompson. [ 1 ] [ 10 ] It is widely accepted now that many if not most behaviours in animals and humans are under significant genetic influence, although the extent of genetic ...
For fields such as social and behavioral genomics that are shaped by an ugly history and uncertain future, socially and ethically responsible research and research communication are crucial.
The most important development during this century of behavioral genetic research has been the synthesis of the two worlds of genetics, quantitative genetics and molecular genetics. Quantitative genetics and molecular genetics both have their origins in the 1860s with Francis Galton (Galton 1865 , 1869 ) and Gregor Mendel (Mendel 1866 ...
Jan 1, 2008 · A systematic review of contemporary behavioral genetic research is well beyond the scope of the present article. As an alternative, we illustrate the focus of contemporary behavioral genetic research on joint models of genetic and environmental effects by describing selected findings from the MCTFR as well as related findings from the larger field.
Apr 14, 2021 · In this introductory chapter, we discuss the nexus between evolutionary theory and behavioral genetics, using it to elucidate the biological origins of human behavior and motivational predispositions.
Mar 3, 2023 · A century after the first twin and adoption studies of behavior in the 1920s, this review looks back on the journey and celebrates milestones in behavioral genetic research. After a whistle-stop tour of early quantitative genetic research and the parallel journey of molecular genetics, the travelogue focuses on the last fifty years.
Feb 9, 2023 · Behavioural genetics examines the underlying factors associated with individual differences in behaviours and capacities. In this Primer, Willoughby et al. discuss the methods used in behavioural ...
Behavioral genetics research indicates that genetic factors play a role in individual differences in children’s temperaments. In this work, researchers use genetically informative samples (e.g., twins or adoptive/nonadoptive siblings) to decompose the observed (i.e., phenotypic) variance of a temperament trait into genetic, shared, and nonshared environmental variance components.